We have discussed mutational burden previously on this blog – in essence, the concept is that tumors with more mutations are more visible to the immune system because the generation of new novel antigenic epitopes allows for adaptive immune responses even when previous adaptive antigen-specific immune responses have been blunted by PD-1 expression. Continue reading
Tag Archives: Pembrolizumab
Adenosine – a critical checkpoint in the tumor microenvironment
The tumor microenvironment (TME) includes a host of cells (mesenchymal, immune, vascular), cytokines, and other signaling molecules that serve to abrogate the innate and adaptive immune responses against the tumor. This is appropriate to maintain tissue homeostasis, and to prevent autoimmunity after the rogue cancer cells have been eliminated. Cancer cells co-opt many of these pathways to terminate the effective immune response so that they are not wiped out, rather, proliferate, invade, metastasize and kill, Continue reading
Pancreatic cancer – early detection, immune response, and infection-based resistance
Approximately 1.6 percent of men and women will be diagnosed with pancreatic cancer at some point during their lifetime. In 2014, an estimated 64,668 patients were living with the disease. The five-year survival for pancreatic cancer is 8.2% and it is projected to be the second leading cause of death due to cancer (behind lung cancer) in the US by the year 2030. For good reason, then, November is Pancreatic Awareness Month. Several recent research items are of particular interest to us. Continue reading
New Link’s Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading
Recent immune checkpoint study failures do not dampen enthusiasm for the future
Immune checkpoint inhibitors are simply cancer wonder drugs about which we are learning more each day. Because they don’t work optimally in many patients and some even hyper-progress, the goal is to determine ways to expand their effectiveness to more patients. As such, the number of clinical studies with checkpoints and checkpoint combinations continues to grow.
Immune checkpoint inhibitors act by blocking the abrogating phase of the immune response that is necessary to prevent autoimmune disease – by prolonging the immune response against cancer, a more robust and prolonged immune response, which is required for effective cancer therapy, is achieved with checkpoint therapy. Continue reading
Imfinzi, the latest approved checkpoint, and checkpoint combinations
The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
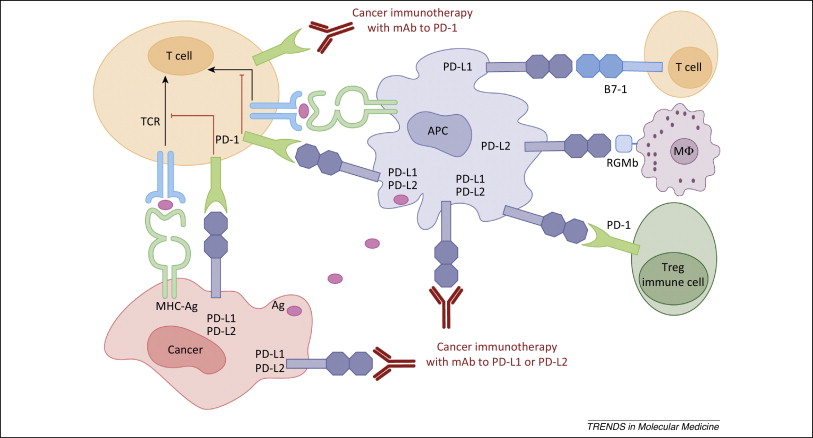
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
TIGIT, a CTLA4-esque Immune Checkpoint for Cancer
Immune checkpoint-directed therapy is producing unprecedented clinical results in many patients. So much so, that the FDA recently reversed its longstanding policy or approving cancer drugs based on site of origin, to the presence of a biomarker (microsatellite instability (MSI-H) or mismatch-deficient repair (dMDR) as the indication for therapy with pembrolizumab (Ketruda), and PD-1 blocker. Cancers expressing MSI-H or dMDR mutate at a rapid rate, presenting novel epitopes to the immune system, which is readily mobilized against them so that tumor infiltrating T-cells are reliably present. Blocking the PD-1/PD-L1 pathway in this context allows for prolongation of the immune response and better clinical results. Continue reading
PD-L1 Inhibitor, avelumab, approved for Merkel cell carcinoma
Avelumab (Bavencio) is a PD-L1 inhibitor that was approved for the treatment of patients with metastatic Merkel cell carcinoma (MCC). Continue reading
Hyperprogression on Checkpoint Inhibition Immunotherapy
Results with checkpoint inhibitors nivolumab (PD-1, Opdivo), pembrolizumab (PD-1, Keytruda), and atezolizumab (PD-L1, Tecentriq) are impressive. Some patients have experienced incredible and prolonged responses. These drugs are truly modern medical breakthroughs.
Continue reading
PD-L1 Expression correlates with outcomes in patients with melanoma
Keytruda (pembrolizumab) and Opdivo (nivolumab) are monoclonal antibodies that disrupt the PD-1 (Opdivo) / PD-L1 (Keytruda) pathway; they are approved by the FDA for the treatment of patients with unresectable melanoma, as well as other cancers including non-small cell lung cancer (NSCLC), head and neck cancer, renal cell carcinoma (Opdivo), and Hodg kin lymphoma (Opdivo). Keytruda is limited to patients with NSCLC with a tumor proportion score (TPS) of greater than 50% for PD-L1 staining. Continue reading
