The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
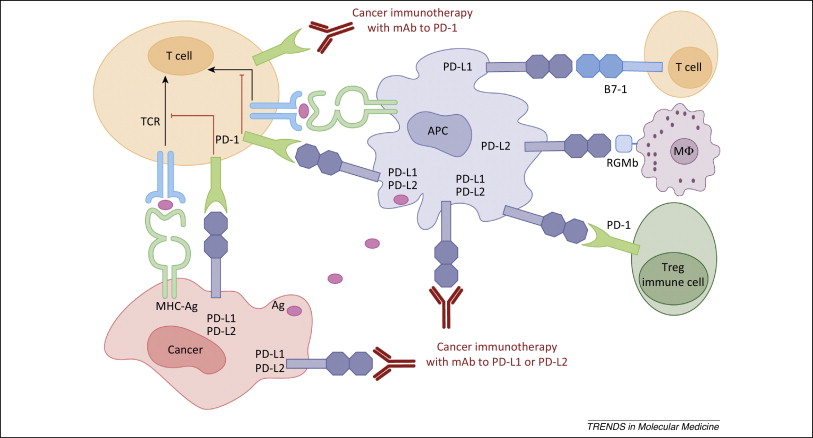
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
