We have discussed mutational burden previously on this blog – in essence, the concept is that tumors with more mutations are more visible to the immune system because the generation of new novel antigenic epitopes allows for adaptive immune responses even when previous adaptive antigen-specific immune responses have been blunted by PD-1 expression. Continue reading
Category Archives: Mutations
Using a blood test to select patients most likely to respond to checkpoint therapy
Checkpoint therapy with PD-(L)1 and CTLA4-directed monoclonal antibodies has shown to be extremely effective for many patients with a variety of tumors. PD-1 testing, alone, however, are lacking in selecting patients for therapy – up to 17% of patients who do not meet criteria for PD-1 positivity respond to treatment, and many patients with PD-1 tumors do not respond well to checkpoint therapy. Continue reading
Sitravatinib plus nivolumab in NSCLC
Sitravatinib (MGCD516) is an oral multi-tyrosine kinase inhibitor being developed by Mirati Therapeutics. Last week, the company announced that three of eleven patients with non-small cell lung cancer (NSCLC) with genetic alterations in MET, AXL, RET, TRK, DDR2, KDR, PDGFRA, KIT or CBL who were resistant to checkpoint [anti PD-(L)1 therapy] had confirmed partial responses; because of this, dosing in the 34-patient expansion cohort will proceed. Continue reading
ALK-positive lung cancer – antibodies to fusion protein
Approximately 7% of patients with non-small cell lung cancer (NSCLC) possess a transgene that results from an inversion of chromosome 2 that juxtaposes the 5’ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the anaplastic lymphoma kinase (ALK) gene, resulting in the novel fusion oncogene EML4-ALK . Continue reading
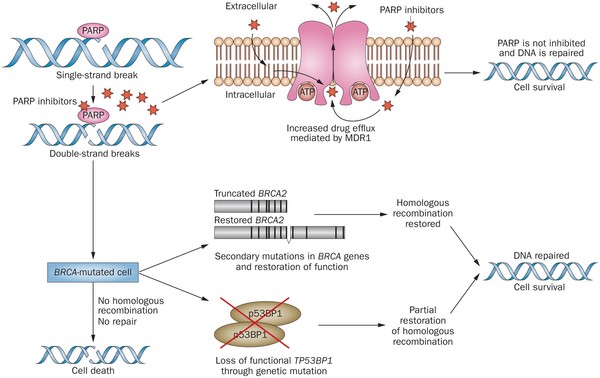
Olaparib – PARP inhibitor for triple negative breast cancer
Olaparib (Lynparza) is a PARP (poly-ADP ribose polymerase) inhibitor that was approved by the FDA in 2014 for the treatment of patients with advanced ovarian cancer who have mutated BRCA1,2 gene. Recently, the drug showed a 70% reduction in risk of progression in patients with less-advanced disease in the maintenance therapy setting:
The Phase III SOLO-2 trial demonstrated a significant improvement in progression-free survival (PFS) in germline BRCA-mutated (gBRCA), platinum-sensitive, relapsed ovarian cancer patients treated with Lynparza (olaparib) tablets (300mg twice daily) compared with placebo in the maintenance setting. The trial met its primary endpoint of investigator assessed PFS (HR 0.30; 95% CI 0.22 to 0.41; P<0.0001; median 19.1 months vs 5.5 months).
PARP inhibitors act in a counter-intuitive manner – by blocking PARP in the context of mutated BRCA1, the cell becomes overwhelmed with double strand breaks, leading to crisis and cell death. BRCA1 mutations, alone, predispose the cell to the accumulation of mutations in protooncogenes and tumor suppressor genes – a few double strand breaks are tumorigenic, whereas a massive number of double strand breaks, as occurs in the context of PARP inhibition, leads to apoptosis.
The use of PARP inhibitors for breast cancer makes great sense, However, in a Phase 3 trial of velparib, an experimental PARP inhibitor, failed to achieve better rates of complete pathogenic response in patients with triple negative breast cancer (TNBC – lack of HER-2, estrogen, and progesterone receptor up-regulation) versus chemotherapy, alone.
At the ASCO conference last week, AstraZeneca presented data on the use of olaparib in 302 patients with BRCA1,2 mutated breast cancer from its OlympiAD trial that compares olaparib against physician’s choice of chemotherapy (capecitabine 2500 mg/m2 d1-14 q 21, or vinorelbine 30 mg/m2 d1,8 q 21, or eribulin 1.4 mg/m2 d1,8 q 21):
OlympiAD Inclusion Criteria:
- Germline mutation in BRCA1 or BRCA2 that is predicted to be deleterious or suspected deleterious.
- Histologically or cytologically confirmed breast cancer with evidence of metastatic disease.
- Prior therapy with an anthracycline and a taxane in either an adjuvant or metastatic setting.
- Prior platinum allowed as long as no breast cancer progression occurred on treatment or if given in adjuvant/neoadjuvant setting at least 12 months from last dose to study entry elapsed.
- ER/PR breast cancer positive patients must have received and progressed on at least one endocrine therapy (adjuvant or metastatic), or have disease that the treating physician believes to be inappropriate for endocrine therapy.
- ECOG performance status 0-1.
- Adequate bone marrow, kidney and liver function.
OlympiAD Exclusion Criteria:
- Prior treatment with PARP inhibitor.
- Patients with HER2 positive disease.
- More than 2 prior lines of chemotherapy for metastatic breast cancer.
- Untreated and/or uncontrolled brain metastases.
Results were quite impressive – this was the first study that demonstrated PARP inhibition is effective in breast cancer:
- About 60% of patients saw their tumors shrink, a hair more than double the 29% objective response rate seen in those patients on chemotherapy.
- Lynparza showed efficacy in patients with TNBC, which is more difficult to treat. AbbVie, which is developing its own PARP inhibitor called veliparib, recentlyannounced a study specifically geared to look at veliparib’s activity in triple negative breast cancer failed to show a benefit when added to chemo.
- Additionally, treatment with Lynparza improved the time to second progression or death compared to chemo,suggesting patients who relapsed after Lynparza experienced a less aggressive return of their cancers.
Astrazeneca is studying olaparib with many combinations, including a study in TNBC with PD-L1 inhibitor durvalumab and CTLA-4 inhibitor tremelimumab.
Regorafenib approved for hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is a primary cancer of the liver that occurs as a result of chronic liver disease, including cirrhosis and hepatitis B and C infections (Figure 1). Serum alpha-fetoprotien (AFP) levels are elevated early in the disease, and screening of patients with chronic liver disease for AFP has lead to earlier diagnosis of HCC. The test is 40-64% sensitive (the ability to detect disease when disease is truly present) because many HCCs do not produce AFP, but it is 75-91% specific (the ability to rule out disease when disease is truly absent) – an AFP of over 400 mg/mL is considered diagnostic. Continue reading
PD-L1 Inhibitor, avelumab, approved for Merkel cell carcinoma
Avelumab (Bavencio) is a PD-L1 inhibitor that was approved for the treatment of patients with metastatic Merkel cell carcinoma (MCC). Continue reading
Hyperprogression on Checkpoint Inhibition Immunotherapy
Results with checkpoint inhibitors nivolumab (PD-1, Opdivo), pembrolizumab (PD-1, Keytruda), and atezolizumab (PD-L1, Tecentriq) are impressive. Some patients have experienced incredible and prolonged responses. These drugs are truly modern medical breakthroughs.
Continue reading
CDK4/6 Inhibitors – Excellent Results Support Paradigm Shift in HR+/HER2- Breast Cancer
We have previously written about CDK4/6 inhibitor, palbociclib (Ibrance) for the treatment of patients with hormone receptor positive (HR+) Her-2 negative (HER2-) disease. In a randomized study of 165 patients who had not been treated previously, palbociclib plus letrozole (a nonsteroidal competitive inhibitor of the aromatase enzyme system that inhibits the conversion of androgens to estrogens) was superior to letrozole, alone: Continue reading
Tagrisso is superior to platinum-based chemotherapy for patients with relapsed lung cancer following front-line anti EGFR therapy
Tagrisso (osimertinib) is a kinase inhibitor indicated for patients with metastatic epidermal growth factor T790M mutation-positive lung cancer. It was approved under accelerated approval provisions in November 2015 on the basis of phase 2 trials demonstrating a combined overall objective response rate of 59%. Continue reading