BRCA1/2 are DNA repair enzymes that are the essential components of homologous repair (HR), which is invoked when DNA doubles strand breaks are encountered. HR works via consultation of sister chromatids during late S to early G2 of the cell cycle – without repair of double strand breaks, mitotic catastrophe ensues: Continue reading
Tag Archives: BRCA1
Olaparib – PARP inhibitor for triple negative breast cancer
Olaparib (Lynparza) is a PARP (poly-ADP ribose polymerase) inhibitor that was approved by the FDA in 2014 for the treatment of patients with advanced ovarian cancer who have mutated BRCA1,2 gene. Recently, the drug showed a 70% reduction in risk of progression in patients with less-advanced disease in the maintenance therapy setting:
The Phase III SOLO-2 trial demonstrated a significant improvement in progression-free survival (PFS) in germline BRCA-mutated (gBRCA), platinum-sensitive, relapsed ovarian cancer patients treated with Lynparza (olaparib) tablets (300mg twice daily) compared with placebo in the maintenance setting. The trial met its primary endpoint of investigator assessed PFS (HR 0.30; 95% CI 0.22 to 0.41; P<0.0001; median 19.1 months vs 5.5 months).
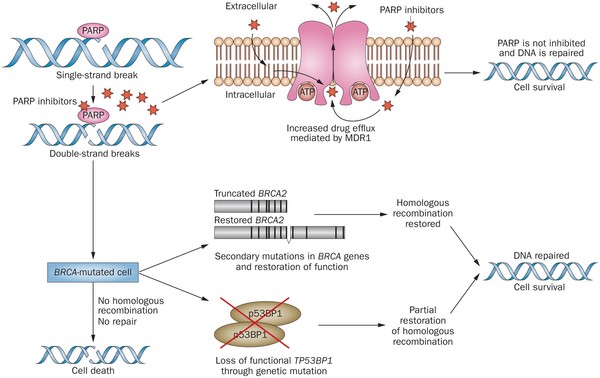
PARP inhibitors act in a counter-intuitive manner – by blocking PARP in the context of mutated BRCA1, the cell becomes overwhelmed with double strand breaks, leading to crisis and cell death. BRCA1 mutations, alone, predispose the cell to the accumulation of mutations in protooncogenes and tumor suppressor genes – a few double strand breaks are tumorigenic, whereas a massive number of double strand breaks, as occurs in the context of PARP inhibition, leads to apoptosis.
The use of PARP inhibitors for breast cancer makes great sense, However, in a Phase 3 trial of velparib, an experimental PARP inhibitor, failed to achieve better rates of complete pathogenic response in patients with triple negative breast cancer (TNBC – lack of HER-2, estrogen, and progesterone receptor up-regulation) versus chemotherapy, alone.
At the ASCO conference last week, AstraZeneca presented data on the use of olaparib in 302 patients with BRCA1,2 mutated breast cancer from its OlympiAD trial that compares olaparib against physician’s choice of chemotherapy (capecitabine 2500 mg/m2 d1-14 q 21, or vinorelbine 30 mg/m2 d1,8 q 21, or eribulin 1.4 mg/m2 d1,8 q 21):
OlympiAD Inclusion Criteria:
- Germline mutation in BRCA1 or BRCA2 that is predicted to be deleterious or suspected deleterious.
- Histologically or cytologically confirmed breast cancer with evidence of metastatic disease.
- Prior therapy with an anthracycline and a taxane in either an adjuvant or metastatic setting.
- Prior platinum allowed as long as no breast cancer progression occurred on treatment or if given in adjuvant/neoadjuvant setting at least 12 months from last dose to study entry elapsed.
- ER/PR breast cancer positive patients must have received and progressed on at least one endocrine therapy (adjuvant or metastatic), or have disease that the treating physician believes to be inappropriate for endocrine therapy.
- ECOG performance status 0-1.
- Adequate bone marrow, kidney and liver function.
OlympiAD Exclusion Criteria:
- Prior treatment with PARP inhibitor.
- Patients with HER2 positive disease.
- More than 2 prior lines of chemotherapy for metastatic breast cancer.
- Untreated and/or uncontrolled brain metastases.
Results were quite impressive – this was the first study that demonstrated PARP inhibition is effective in breast cancer:
- About 60% of patients saw their tumors shrink, a hair more than double the 29% objective response rate seen in those patients on chemotherapy.
- Lynparza showed efficacy in patients with TNBC, which is more difficult to treat. AbbVie, which is developing its own PARP inhibitor called veliparib, recentlyannounced a study specifically geared to look at veliparib’s activity in triple negative breast cancer failed to show a benefit when added to chemo.
- Additionally, treatment with Lynparza improved the time to second progression or death compared to chemo,suggesting patients who relapsed after Lynparza experienced a less aggressive return of their cancers.
Astrazeneca is studying olaparib with many combinations, including a study in TNBC with PD-L1 inhibitor durvalumab and CTLA-4 inhibitor tremelimumab.

The Roles of P53, BRCA1, and PTEN in Hereditary Cancers – Lauren Fitzgerald, Contributor
Cancer results from accumulated mutations in the cancer cell’s genome. These mutations can occur spontaneously in any cell throughout an individual’s lifetime, often increasing with age or exposure to carcinogenic or mutagenic compounds. These are called somatic mutations that do not exist in every cell, and cannot be passed along from one generation to the next. However, in approximately 5 to 10% of all cancer cases, mutations are passed along through the germ line and can predispose an individual to various types of cancers. Continue reading

ROCA Ovarian Cancer Test for Early Detection
Each year, about 20,000 women in the United States get ovarian cancer. Among women in the United States, ovarian cancer is the eighth most common cancer and the fifth leading cause of cancer death, after lung and bronchus, breast, colorectal, and pancreatic cancers. In 2012 (the most recent year numbers are available)— 20,785 women in the United States were diagnosed with ovarian cancer, and 14,404 women in the United States died from ovarian cancer. Continue reading
PARP inhibitor rejected by FDA Advisory Committee, then Approved for refractory patients
In June 2014, the FDA Oncology Drug Advisory Committee voted 11-2 to delay approval of AstraZeneca’s olaparib, a PARP (poly-ADP ribose polymerase) inhibitor for maintenance therapy in patients with ovarian cancer. The product received Accelerate Approval designation from the FDA, which provides for conditional approval pending follow-up studies, based on surrogate endpoints from Phase 2 trials, in this case, progression-free survival (PFS). Continue reading