We have previously written about CDK4/6 inhibitor, palbociclib (Ibrance) for the treatment of patients with hormone receptor positive (HR+) Her-2 negative (HER2-) disease. In a randomized study of 165 patients who had not been treated previously, palbociclib plus letrozole (a nonsteroidal competitive inhibitor of the aromatase enzyme system that inhibits the conversion of androgens to estrogens) was superior to letrozole, alone:
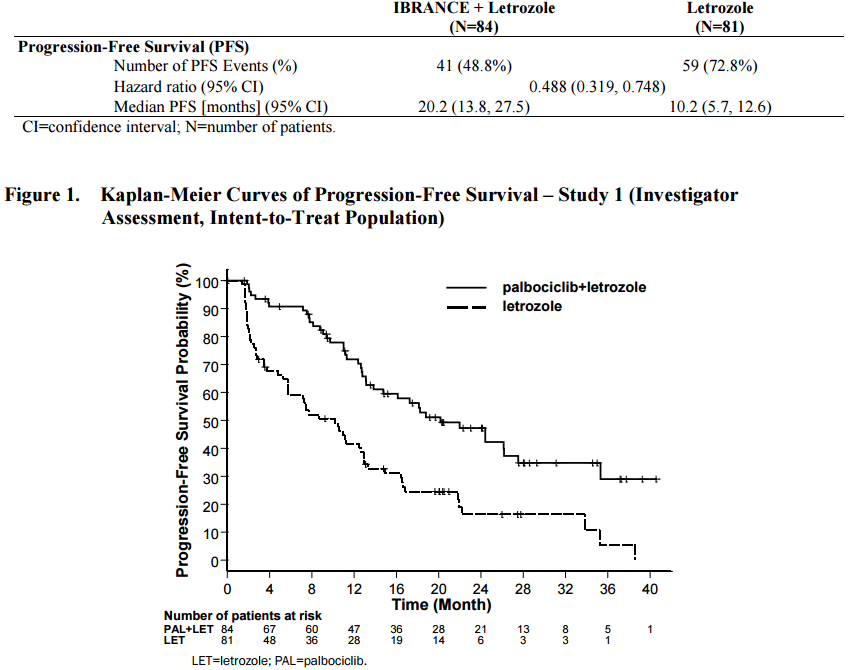
Figure 1. Palbociclib (Ibrance) plus letrozole (Femara) versus palbociclib, alone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207103s000lbl.pdf
Similar studies in HR+/HER2- breast cancer patients with ribociclib from Novartis, demonstrate a class effect – MONALEESA-2 found a 44% reduction in the hazard for progression or death in women with advanced hormone receptor (HR)-positive/HER2-negative breast cancer treated with ribociclib plus letrozole versus letrozole alone.
The median progression-free survival (PFS) had yet to be reached in the ribociclib group but was “expected to far exceed” the 14.7-month median among patients treated with letrozole alone, as reported by principal investigator Gabriel Hortobagyi, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Data were presented at the ESMO (European Society for Medical Oncology) meeting in October:
Researchers randomized 668 postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer, who had not undergone any prior systemic treatment, to ribociclib (600 mg/day, 3 weeks on/1 week off) and letrozole (2.5 mg/day, continuous), or letrozole plus placebo.
In the ribociclib arm, there was a 44% improvement in the primary objective of progression-free survival compared to the placebo arm (HR: 0.556, p = 0.00000329). Median progression-free survival was 14.7 months in the placebo arm, but was not reached in the ribociclib arm at data cut-off.
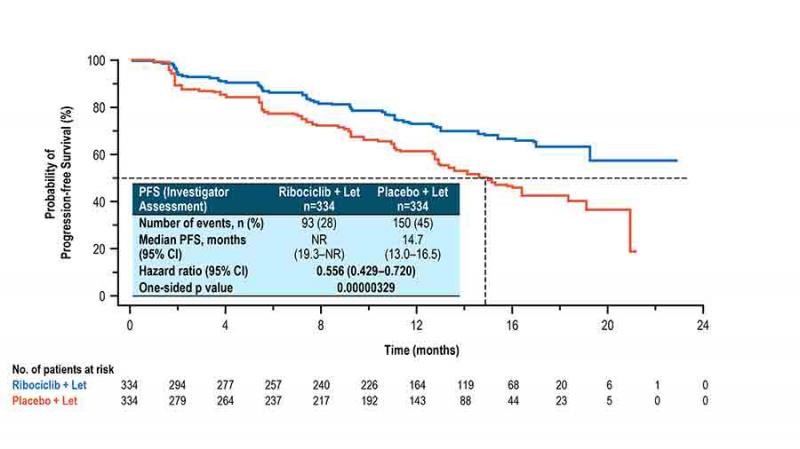
Figure 2. Ribociclib (Ibrance) plus letrozole (Femara) versus ribociclib, alone. http://www.esmo.org/Conferences/ESMO-2016-Congress/Press-Media/Ribociclib-Improves-Progression-free-Survival-in-Advanced-Breast-Cancer
“The results of this trial represent a compelling proof of principle, and suggest a paradigm shift in metastatic, HR+ breast cancer. They also suggest that testing combinations of ribociclib with other inhibitors of various signaling pathways might lead to additional progress in the management of several subtypes of breast cancer,” Hortobagyi said.
Patients with measurable disease at baseline showed a significantly higher objective response rate to ribociclib plus letrozole compared to letrozole alone (53% vs. 37%; p=0.00028), and improved clinical benefit rate (80% vs. 72% p=0.02).
Since its approval in 2015, widespread uptake of Ibrance (palbociclib) in front-line treatment of with HR+/HER2- breast cancer patients. It received expanded approval in 2016 for use in combination with Faslodex (fulvestrant, a competitive antagonist of the estrogen receptor) for patietns with HR+/HER2- breast cancer that failed initial anti-estrogen therapy.
“With respect to breast cancer, I think doctors have been very persuaded by the results with these drugs,” said Harold Burstein, MD, PhD, of Dana-Farber Cancer Institute in Boston. “There have been no fewer than four major randomized trials that have reported positive results with these drugs, including three with palbociclib.
Burstein predicted that use of CDK4/6 inhibitors will expand beyond current indications into other types of breast cancer (notably, HER2-positive disease), as well as to malignancies other than breast cancer. In particular, the drugs could offer benefits to patients with various types of indolent malignancies, such as neuroendocrine tumors, low-risk prostate cancer, and mantle-cell lymphoma.
