MET is a gene that encodes a receptor tyrosine kinase that is activated upon binding with hepatocyte growth factor (HGF, or Scatter Factor). Specifically, MET is a Continue reading
Tag Archives: NSCLC
ALK-positive lung cancer – antibodies to fusion protein
Approximately 7% of patients with non-small cell lung cancer (NSCLC) possess a transgene that results from an inversion of chromosome 2 that juxtaposes the 5’ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the anaplastic lymphoma kinase (ALK) gene, resulting in the novel fusion oncogene EML4-ALK . Continue reading
PD-L1 Inhibitor, avelumab, approved for Merkel cell carcinoma
Avelumab (Bavencio) is a PD-L1 inhibitor that was approved for the treatment of patients with metastatic Merkel cell carcinoma (MCC). Continue reading
Osteopontin – a prognostic marker for cancer progression
Osteopontin (OPN) is a matrix protein that is expressed by osteoclasts, osteoblasts, dendritic cells, fibroblasts, hepatocytes, smooth and skeletal muscle cells, endothelial cells, and kidney cells. It interacts with many cell surface receptors including integrins and CD44. One of the major physiologic functions of OPN is the control of bone mineralization – by binding to specific apatitie crystal faces, it inhibits mineralization. But, OPN is also a pro-inflammatory cytokine that acts in many tissues to recruit monocytes and macrophages and induce cytokine secretion from leukocytes. As such, it has a critical role in tissue remodeling, inflammation, and tumorigenesis. Continue reading
PD-L1 Expression correlates with outcomes in patients with melanoma
Keytruda (pembrolizumab) and Opdivo (nivolumab) are monoclonal antibodies that disrupt the PD-1 (Opdivo) / PD-L1 (Keytruda) pathway; they are approved by the FDA for the treatment of patients with unresectable melanoma, as well as other cancers including non-small cell lung cancer (NSCLC), head and neck cancer, renal cell carcinoma (Opdivo), and Hodg kin lymphoma (Opdivo). Keytruda is limited to patients with NSCLC with a tumor proportion score (TPS) of greater than 50% for PD-L1 staining. Continue reading
Tagrisso is superior to platinum-based chemotherapy for patients with relapsed lung cancer following front-line anti EGFR therapy
Tagrisso (osimertinib) is a kinase inhibitor indicated for patients with metastatic epidermal growth factor T790M mutation-positive lung cancer. It was approved under accelerated approval provisions in November 2015 on the basis of phase 2 trials demonstrating a combined overall objective response rate of 59%. Continue reading
Rociletinib for Resistant Non-Small Cell Lung Cancer Patients with EGFR T790M Mutation – Anthony J. Meglio, Contributor
There are two major subtypes of lung cancer: Non-Small Cell Lung Cancer (NSCLC), which accounts for 85% of all cases, and Small Cell Lung Cancer (SMLC). About 60% of NSCLC are unresectable at diagnosis, hence, the poor prognosis – ten to twelve months survival when treated with platinum-based chemotherapy. Treatment options are evaluated based on the histologic subtype and the presence of mutations to determine the the best combination of molecular therapies for treatment. Ten to twenty percent of patients with NSCLC have a mutated epidermal growth factor receptor, most commonly. a deletion in the in-frame of exon 19 (around amino acid 747 to 752) or a L858R point mutation of exon 21. On June 1, 2016, the FDA approved the first blood test (liquid biopsy) companion diagnostic to determine whether these mutations are present. Continue reading

Saliva Test to Detect EGFR Mutations and Guide Therapy in Lung Cancer
A new technology called electric field–induced release and measurement (EFIRM) is able to detect biomarkers in saliva for non-small cell lung cancer (NSCLC). The test detects circulating tumor DNA (ctDNA). It is able to detect actionable EGFR (epidermal growth factor receptor) mutations in NSCLC patients with 100% concordance with biopsy-based genotyping, Dr Wong (study author) said, and it can detect the most common EGFR gene mutations that are treatable with TKIs (tyrosine kinase inhibitors), such as gefitinib (Iressa, AstraZeneca Pharmaceuticals LP) or erlotinib (Tarceva, Genentech/Roche). Continue reading

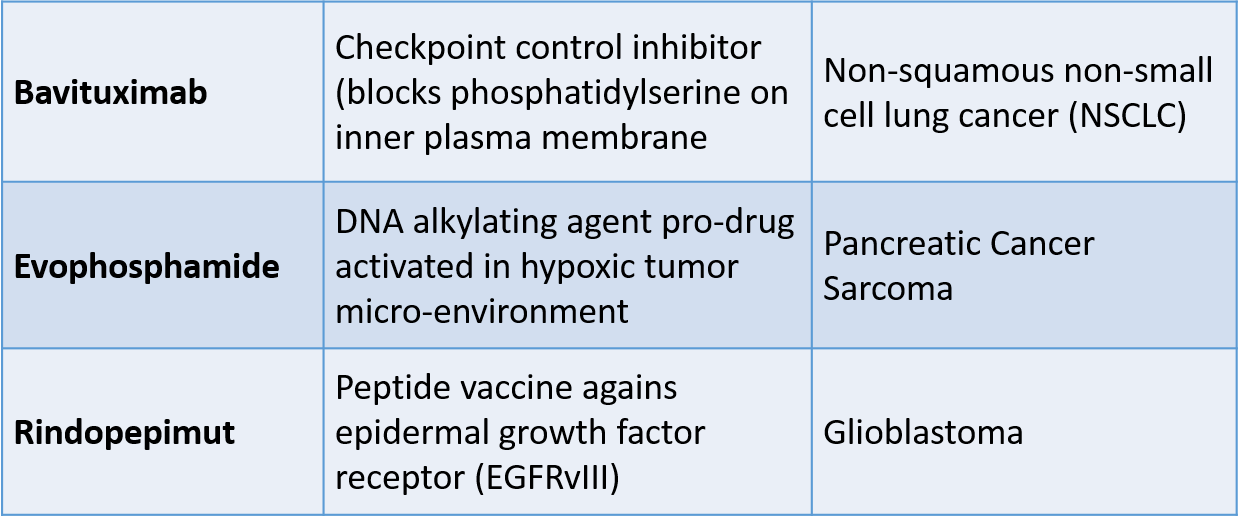
Three Recent Late Stage Disappointments for Lung Cancer, Pancreatic Cancer, Sarcoma, and Glioblastoma
In the last several months, three novel drugs that we have been following on this blog failed in late stage clinical trials. The drugs have diverse mechanisms of action:

Biomarkers for Wnt activation Predict For HER-2(-) Breast Cancer and NSCLC Response to Vantictumab
OncoMed is developing several compounds that target Wnt and Notch pathways, which are important in cancer and cancer stem cell maintenance, survival, and proliferation. Biomarkers for response of Her-2-negative breast cancer and non-small cell lung cancer (NSCLC) following treatment with Vantictumab, anti-Wnt monoclonal antibody, have been developed. Continue reading