Osteopontin (OPN) is a matrix protein that is expressed by osteoclasts, osteoblasts, dendritic cells, fibroblasts, hepatocytes, smooth and skeletal muscle cells, endothelial cells, and kidney cells. It interacts with many cell surface receptors including integrins and CD44. One of the major physiologic functions of OPN is the control of bone mineralization – by binding to specific apatitie crystal faces, it inhibits mineralization. But, OPN is also a pro-inflammatory cytokine that acts in many tissues to recruit monocytes and macrophages and induce cytokine secretion from leukocytes. As such, it has a critical role in tissue remodeling, inflammation, and tumorigenesis.
OPN is encoded by the SPP1 gene, which is comprised of 7 exons and is located on the long arm of chromosome 4 near four other SIBLING (small integrin-binding N-linked glycoproteins) that are contained in bine and dentin – bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE).
OPN is a 34 kDa that undergoes extensive post-translational modification. Three splice variants (OPN-a, OPN-b, and OPN-c) have been identified.
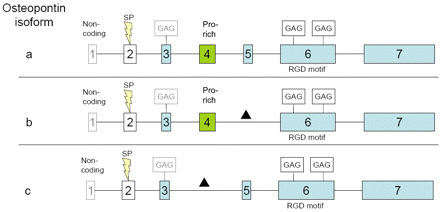
Figure 1. Splice variants of osteopontin (OPN). The exons of the osteopontin gene are highlighted as boxes. a, b and c refer to OPN splice variants a, b and c. Exon 5 and exon 4 are missing in OPN variants b and c, respectively; a corresponds to the full-length protein. Exons marked in blue contain serine-phosphate-rich domains and differential splicing is indicated by a black triangle. SP: Signal peptide; GAG: glycosamino-glycan attachment site; Pro-rich: proline-rich domain; RGD motif: RGD-binding site. http://cgp.iiarjournals.org/content/8/5/211/F5.expansion.html
Role of OPN in tumorigenesis and progression
Evidence that OPN is an important signaling agent in the development and progression of a variety of cancers include:
- OPN-a and OPN-b are expressed in both non-tumor and tumor ovarian samples, but OPN-c is expressed solely in ovarian cancer samples. OPN-c protumorigenic effects are mediated through PI3k/Akt signaling. OPN-c activates OvCar-3 cell proliferation, migration, invasion, anchorage-independent growth, and tumor formation.
- MMP-9 (matrix metalloproteinase 9) cleaves OPN-c, thereby releasing a 5 kDa fragment that binds to CD44 receptors and induces hepatocellular carcinoma invasion and metastasis.
- Independent of HIFα, hypoxia induces OPN expression in breast cancer cells, which leads to NFkB activation, VEGF expression, and angiogenesis.
- OPN synergizes with signaling through the EGFR (epidermal growth factor) and HGF (hepatocyte growth factor).
- OPN induces the expression of oxidoreductases, which protects cells from anoikis during anchorage independent growth.
- OPN induces gene expression changes in a model of breast cancer that reflect the six hallmarks of cancer.
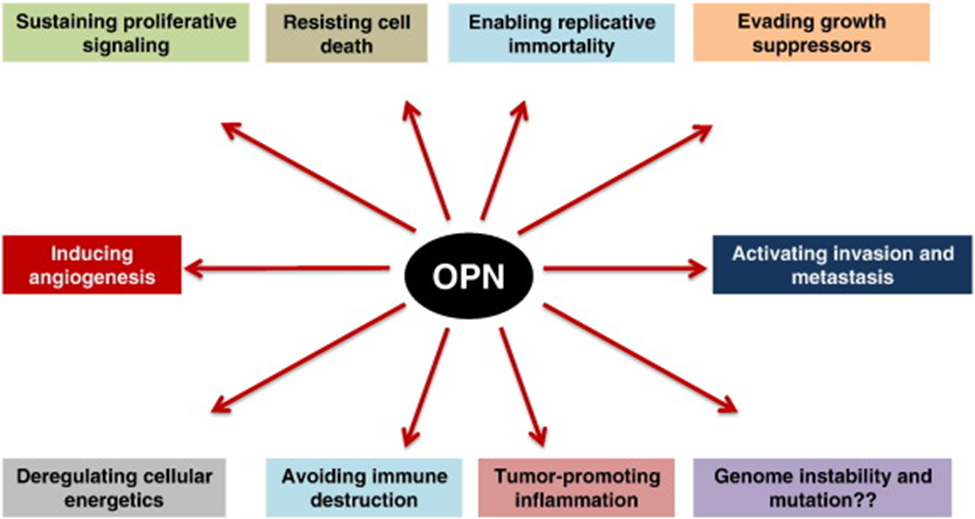
Fig. 2. OPN influences all the six hallmarks of cancer, as well as three of the enabling characteristics of cancer. As of this time there is no data to clearly implicate a role for OPN in genomic instability and mutation. http://www.sciencedirect.com/science/article/pii/S0945053X14000444
Potential of OPN in the management and treatment of cancer
There are two ways that OPN can be exploited – (1) diagnostic/prognostic biomarker, and (2) therapeutic target.
Because OPN is a secreted protein, circulating levels can be measured in blood and used to evaluate prognosis and guide treatment:
- In a study of 158 women with newly diagnosed breast cancer during which serial blood samples were taken, OPN levels over time were strongly associated with poor survival.
- In 27 patients with NSCLC (non-small cell lung cancer), OPN levels at the completion of radiation, as well as the change in plasma concentration during therapy, was predictive of overall survival.
- In a meta-analysis of more than 1,000 patients, OPN expression was a predictor of worse overall survival and disease-free survival/progression-free survival.
- In a retrospective study of 171 patients with NSCLC, OPN and thrombospondin-1 (a matrix protein that induces proliferating endothelial cells to secrete Fas ligand, which mediates apoptosis) levels were compared. OPN levels were inversely associated with survival – for each 50-unit increment in serum OPN, an increased risk of metastasis by 69% and an increased risk of 95% were observed. Conversely, for each 10-unit increment in TSP-1, the risk of death dropped 85%.
Small peptides within the range of the OPN-5 kDa fragment that induced hepatocellular carcinoma cells blocked the CD44-mediated invasion of these cells. Therefore, the development of a small peptide that antagonizes OPN-c may be an appropriate therapeutic strategy.
