The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
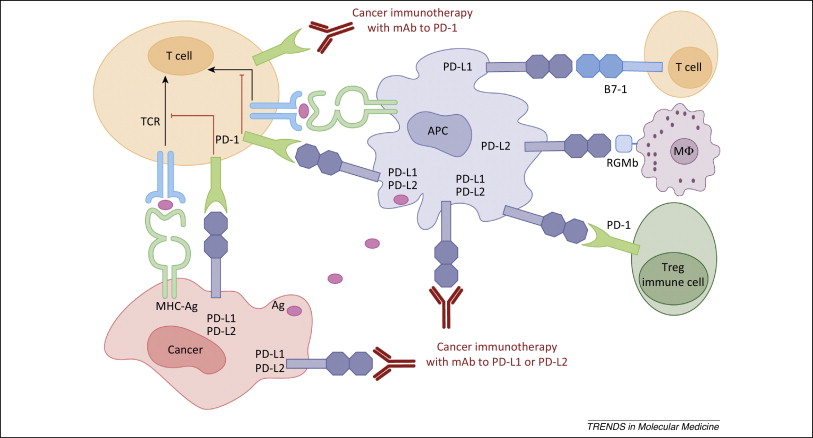
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
It received the FDA nod in May 2017 for the treatment of advanced bladder cancer, regardless of PD-L1 status:
- 182 patients with locally advanced or metastatic urothelial carcinoma were enrolled. Patients had progressed while on or after a platinum-based therapy, including those who progressed within 12 months of receiving therapy in a neo-adjuvant or adjuvant setting
- median age was 67 years (range: 34 to 88), 72% were male, 64% were Caucasian. Sixty-six percent (66%) of patients had visceral metastasis (bone, liver, or lung), including 34% with liver metastasis. Lymph node only metastasis were present in 13% of patients. Sixty-six percent (66%) of patients had ECOG score of 1 and 41% of patients had a baseline creatinine clearance of <60 mL/min. The Bellmunt risk score (which includes ECOG score, baseline hemoglobin, and liver metastases) was 0 in 23%, 1 in 38%, 2 in 29%, and 3 in 9% of patients. Twenty percent (20%) of patients had disease progression following platinum-containing neo-adjuvant or adjuvant chemotherapy as their only prior line of therapy. Seventy percent (70%) of patients received prior cisplatin, 30% prior carboplatin and 35% received ≥2 prior lines of systemic therapy
- Tumor specimens were evaluated prospectively for PD-L1 expression on tumor cells (TC) and immune cells (IC) at a central laboratory using the VENTANA PD-L1 (SP263) Assay. Of the 182 patients, 95 were classified as PD-L1 high (if ICs involve >1% of the tumor area, TC ≥25% or IC ≥25%; if ICs involve ≤1% of the tumor area, TC ≥25% or IC=100%), 73 as PD-L1 low/negative (did not meet criterion for PD-L1 high), and samples for 14 patients were not evaluable.
- Among the total 31 responding patients, 14 patients (45%) had ongoing responses of 6 months or longer and five patients (16%) had ongoing responses of 12 months or longer.
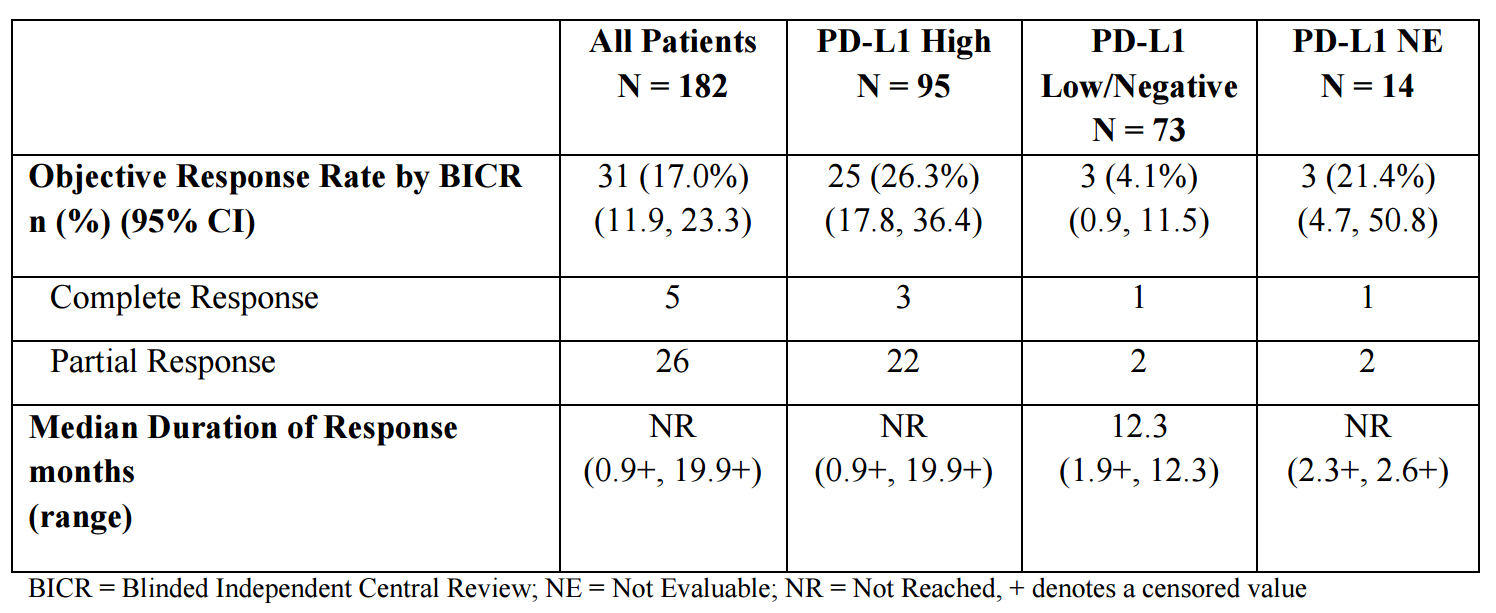
Table 1. Clinical results of durvalumab in bladder cancer. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf
A few weeks after the approval of durvalumab for bladder cancer, excellent clinical results were announced in lung cancer from the Phase 3 PACIFIC trial in patients with locally advanced non-small cell lung cancer (NSCLC) who had not progressed following standard platinum-based chemotherapy concurrent with radiation therapy. The study was stopped at the first planned interim analysis because patients receiving durvalumab had statistically significant increased progression-free survival (PFS). Full data, including overall survival, will be available in the future.
Imfinzi is also being tested in the 1st-line treatment of patients with NSCLC as monotherapy in the MYSTIC and PEARL Phase III trials. It is also being developed in combination with tremelimumab, a checkpoint inhibitor that targets CTLA-4, as part of the MYSTIC, NEPTUNE and POSEIDON Phase III trials.
There are now five approved checkpoint inhibitors targeting the PD-1/PD-L1 axis – Bavencio (avelumab, PD-L1, Pfizer & Merck KGaA: Merkel Cell carcinoma) –Opdivo (nivolumab, PD-1, BMS: melanoma, squamous cell carcinoma of the head and neck – HNSCC, non-small cell lung cancer – NSCLC, bladder cancer, renal cell carcinoma, classical Hodgkin lymphoma), Keytruda (pembrolizumab, PD-1, Merck: melanoma, NSCLC, HNSCC, classical Hodgkin Lymphoma, bladder cancer, Microsatellite Instability-High cancers), and Tecentriq (atrezolizumab, PD-L1, Genentech: metastatic non-small cell lung cancer and bladder cancer).
There are now 756 clinical studies listed on clinicaltrials.gov involving checkpoint combination trials, according to Evaluate EP. Keytruda leads the pack, with 268 — up from 70 just 18 months ago. For Bristol-Myers it’s 242, more than three times the number EP Vantage’s editorial team found in 2015. The trials include PD-1/PD-L1 checkpoints combined with other checkpoints (e.g., IDO inhibitors), cancer vaccines, antibodies to cancer-associated antigens, chemotherapy, kinase inhibitors, and many others.
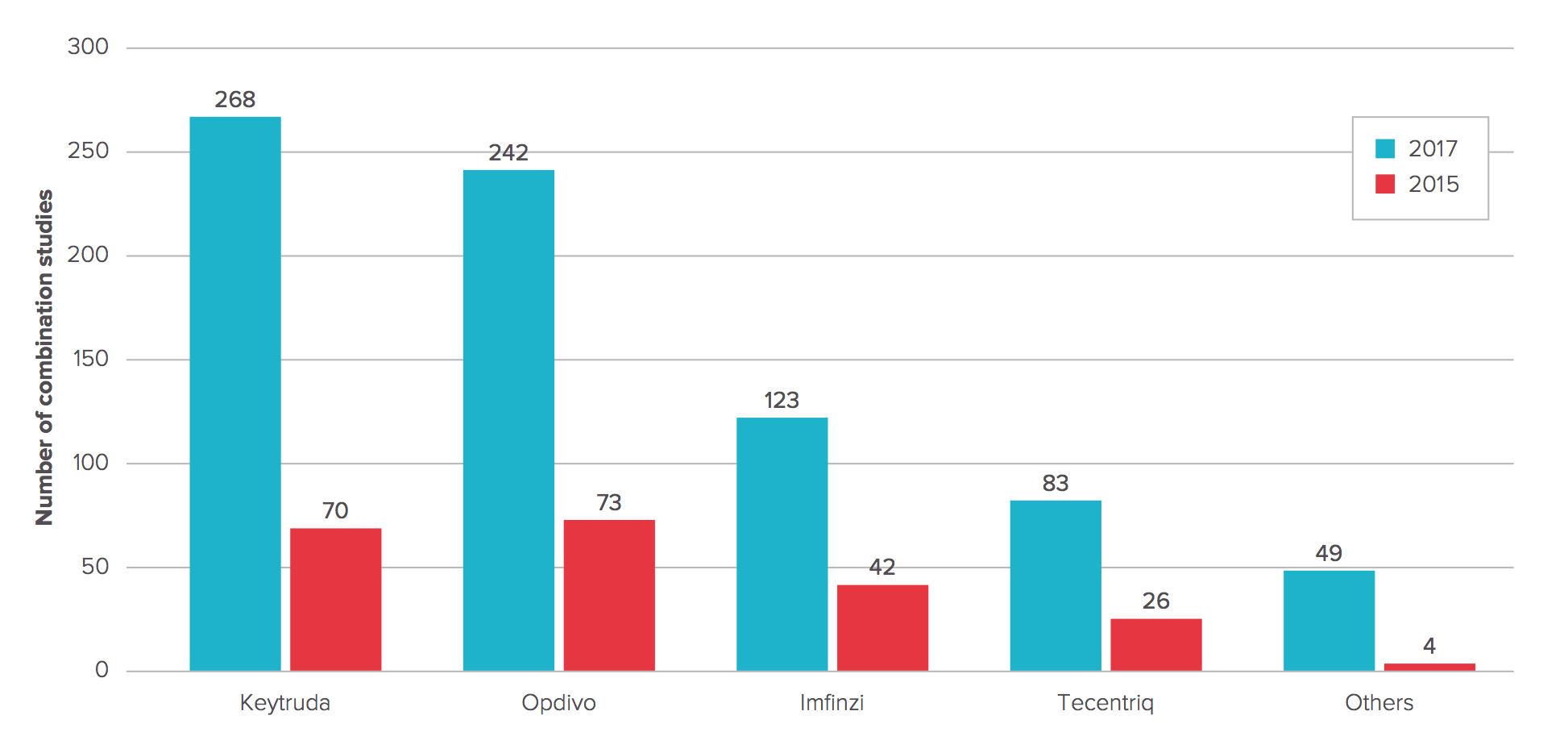
Figure 2. Checkpoint combination trials. http://info.evaluategroup.com/rs/607-YGS-364/images/epv-pdct17.pdf
Given that the first checkpoint inhibitor, Yervoy (ipilimumab, PD-1), and nivolumab and pembrolizumab are indicated for melanoma, there are many combination studies in this disease.

Figure 3. Checkpoint combination trials by cancer type. http://info.evaluategroup.com/rs/607-YGS-364/images/epv-pdct17.pdf
Because anti-CTLA4 and anti-PD-1/PD-L1 antibodies are already on the market, and the great promise of combining these complementary approaches, checkpoint-checkpoint combinations are the most abundant. Indeed, BMS has shown that combining Yervoy and Opdivo provides for significantly enhanced effectiveness in melanoma, but with increased toxicity – forty percent of patients cannot tolerate a full course of therapy. Studies examining how best to combine these agents to maximize effectiveness and mitigate toxicity make great sense. AstraZeneca is also pursuing the combination with durvalumab + its own CTLA-4 inhibitor in development, tremelimumab.

Figure 4. CTLA-4-PD1/PDL1 checkpoint-checkpoint combination studies. http://info.evaluategroup.com/rs/607-YGS-364/images/epv-pdct17.pdf
