AbbVie has recently licensed rights to a monoclonal antibody discovered by Argenx, ARGX-115, paying $20MM up-front and up to $625MM in milestones based on development, approval, and commercial performance. This monoclonal antibody targets a central player in the complex tumor microenvironment – the regulatory T-cell (Treg). Continue reading
Tag Archives: PD-1
Turning “cold tumors hot” – rationale, major setback, and promise
One of the most active fields of clinical investigation in immuno-oncology today is augmenting responses to checkpoint [CTLA4 or PD-(L)1] inhibition therapy by re-sensitizing tumors that were initially unresponsive or had stopped responding to treatment. Continue reading
Mutational burden biomarker – not just mismatch repair deficiency
We have discussed mutational burden previously on this blog – in essence, the concept is that tumors with more mutations are more visible to the immune system because the generation of new novel antigenic epitopes allows for adaptive immune responses even when previous adaptive antigen-specific immune responses have been blunted by PD-1 expression. Continue reading
Adenosine – a critical checkpoint in the tumor microenvironment
The tumor microenvironment (TME) includes a host of cells (mesenchymal, immune, vascular), cytokines, and other signaling molecules that serve to abrogate the innate and adaptive immune responses against the tumor. This is appropriate to maintain tissue homeostasis, and to prevent autoimmunity after the rogue cancer cells have been eliminated. Cancer cells co-opt many of these pathways to terminate the effective immune response so that they are not wiped out, rather, proliferate, invade, metastasize and kill, Continue reading
Priming cancer for immunotherapy
Augmenting the responses to checkpoint inhibitors, which remove the “breaks” from the immune response, is a very popular area of research. The general concept is to turn immunologically cold tumors hot. For example, triple negative breast cancer (TNBC) is considered an immunologically cold tumor – anti-PD(L)1 therapy has shown responses of just 5-10%. Continue reading
Opdivo and Yervoy, the new front-line standard for poor/intermediate-risk renal cell carcinoma
The results of CheckMate 214 demonstrated that combination checkpoint immunotherapy with nivolumab (Opdivo; anti-PD-1 monoclonal antibody) and ipilimumab (Yervoy; anti-CTLA-4 monoclonal antibody), is superior to sunitinib (Sutent; multikinase inhibitor) in the treatment of patients with newly diagnosed renal cell carcinoma (RCC). Interestingly, prior to sunitinib, another immunotherapeutic approach – interferon-alpha (IFN-α) – was the front-line treatment of choice for renal cell carcinoma, which, like melanoma, is very immune-responsive. Continue reading
New Link’s Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading
Imfinzi, the latest approved checkpoint, and checkpoint combinations
The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
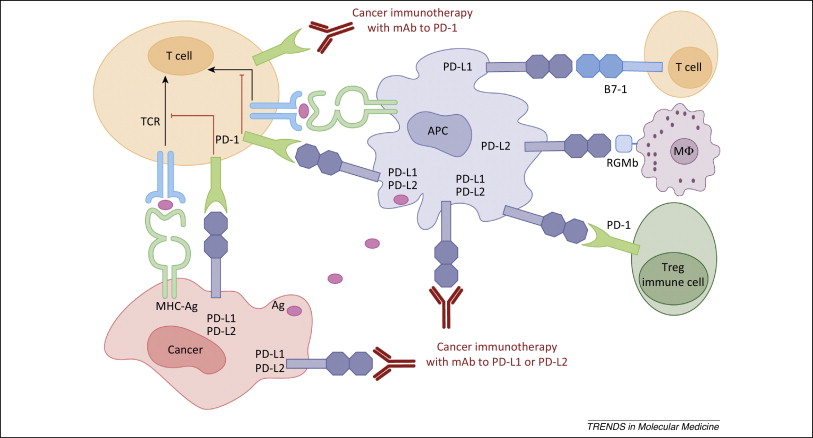
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
BMS and PsoOxus collaborate on transgenic oncolytic virus plus nivolumab
BMS paid PsiOxus $50MM upfront for exclusive rights to develop PsiOxus’ NG-348 enadenotucirev, a systemically administered oncolytic adenovirus therapeutic, in combination with Bristol-Myers Squibb’s Immuno-Oncology checkpoint inhibitor Opdivo (nivolumab) to treat a range of solid tumor types in late-stage cancer patients. This is a “big deal” – PsiOxus could receive up to $886 million in development, regulatory, and sales-based milestones, plus sales royalties. Continue reading
How PD-1 abrogates the anti-tumor immune response
PD-1 inhibition (Figure 1) has quickly become a front-line therapy for non-small cell lung cancer and melanoma. Moreover, PD-1 and PD-L1 inhibitors are being tested in combination with other checkpoint inhibitors, targeted therapies, cancer vaccines, monoclonal antibodies, and other modalities. But, how does PD-1 blunt the anti-tumor immune response? Continue reading
