MET is a gene that encodes a receptor tyrosine kinase that is activated upon binding with hepatocyte growth factor (HGF, or Scatter Factor). Specifically, MET is a Continue reading
Tag Archives: nivolumab
Early discontinuation of checkpoint inhibition due to immune-related side effects does not have a significant impact on treatment efficacy
A course of treatment with checkpoint inhibitors Yervoy (ipilimumab) and Opdivo (nivolumab) for patients with unresectable or metastatic melanoma is every 3 weeks for a total of four doses. Almost forty percent of patients receiving this combined regimen discontinue treatment because of immune-related adverse events. Continue reading
Priming cancer for immunotherapy
Augmenting the responses to checkpoint inhibitors, which remove the “breaks” from the immune response, is a very popular area of research. The general concept is to turn immunologically cold tumors hot. For example, triple negative breast cancer (TNBC) is considered an immunologically cold tumor – anti-PD(L)1 therapy has shown responses of just 5-10%. Continue reading
Sitravatinib plus nivolumab in NSCLC
Sitravatinib (MGCD516) is an oral multi-tyrosine kinase inhibitor being developed by Mirati Therapeutics. Last week, the company announced that three of eleven patients with non-small cell lung cancer (NSCLC) with genetic alterations in MET, AXL, RET, TRK, DDR2, KDR, PDGFRA, KIT or CBL who were resistant to checkpoint [anti PD-(L)1 therapy] had confirmed partial responses; because of this, dosing in the 34-patient expansion cohort will proceed. Continue reading
Opdivo and Yervoy, the new front-line standard for poor/intermediate-risk renal cell carcinoma
The results of CheckMate 214 demonstrated that combination checkpoint immunotherapy with nivolumab (Opdivo; anti-PD-1 monoclonal antibody) and ipilimumab (Yervoy; anti-CTLA-4 monoclonal antibody), is superior to sunitinib (Sutent; multikinase inhibitor) in the treatment of patients with newly diagnosed renal cell carcinoma (RCC). Interestingly, prior to sunitinib, another immunotherapeutic approach – interferon-alpha (IFN-α) – was the front-line treatment of choice for renal cell carcinoma, which, like melanoma, is very immune-responsive. Continue reading
New Link’s Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading
Recent immune checkpoint study failures do not dampen enthusiasm for the future
Immune checkpoint inhibitors are simply cancer wonder drugs about which we are learning more each day. Because they don’t work optimally in many patients and some even hyper-progress, the goal is to determine ways to expand their effectiveness to more patients. As such, the number of clinical studies with checkpoints and checkpoint combinations continues to grow.
Immune checkpoint inhibitors act by blocking the abrogating phase of the immune response that is necessary to prevent autoimmune disease – by prolonging the immune response against cancer, a more robust and prolonged immune response, which is required for effective cancer therapy, is achieved with checkpoint therapy. Continue reading
Neo-antigen vaccines extend progression-free survival in melanoma
Two companies – Biontech, RNA vaccine and Neon Therapeutics, peptide vaccine – reported very positive results of neo-antigen vaccines in patients with refractory metastatic melanoma. Continue reading
Imfinzi, the latest approved checkpoint, and checkpoint combinations
The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
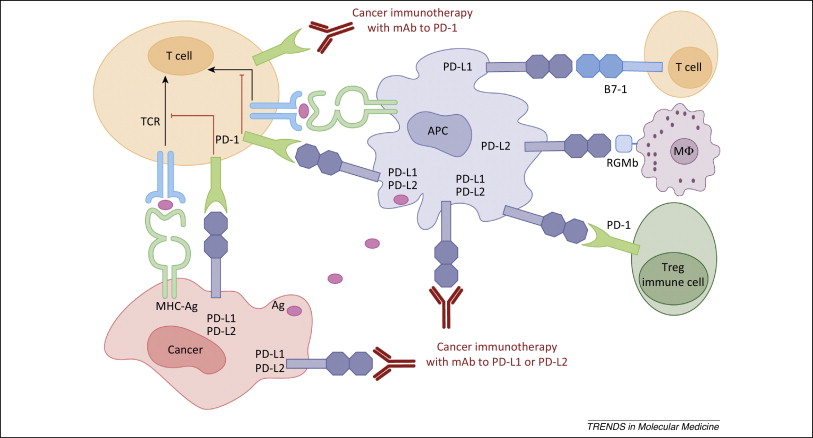
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
Peripheral white blood cell profiles may predict response to checkpoint blockade
While some patients enjoy excellent and durable responses to treatment with checkpoint inhibits, most do not, and some even hyper-progress. Selecting patients who will benefit most has been challenging. Continue reading
