Not very, according to the REACT Study conducted by the Imperial College London. The study was predicated on the importance of COX-2 in driving inflammation that contributes to tumorigenesis. The thinking was that administering celecoxib (Celebrex), a COX-2 inhibitor, could reduce breast cancer progression, as suggested in smaller observational trials. Continue reading
Tag Archives: breast cancer
Recent immune checkpoint study failures do not dampen enthusiasm for the future
Immune checkpoint inhibitors are simply cancer wonder drugs about which we are learning more each day. Because they don’t work optimally in many patients and some even hyper-progress, the goal is to determine ways to expand their effectiveness to more patients. As such, the number of clinical studies with checkpoints and checkpoint combinations continues to grow.
Immune checkpoint inhibitors act by blocking the abrogating phase of the immune response that is necessary to prevent autoimmune disease – by prolonging the immune response against cancer, a more robust and prolonged immune response, which is required for effective cancer therapy, is achieved with checkpoint therapy. Continue reading
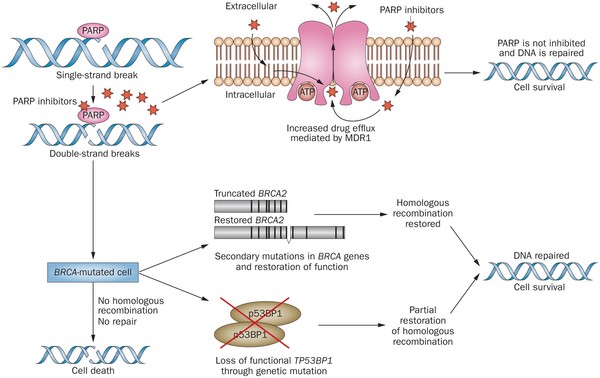
Olaparib – PARP inhibitor for triple negative breast cancer
Olaparib (Lynparza) is a PARP (poly-ADP ribose polymerase) inhibitor that was approved by the FDA in 2014 for the treatment of patients with advanced ovarian cancer who have mutated BRCA1,2 gene. Recently, the drug showed a 70% reduction in risk of progression in patients with less-advanced disease in the maintenance therapy setting:
The Phase III SOLO-2 trial demonstrated a significant improvement in progression-free survival (PFS) in germline BRCA-mutated (gBRCA), platinum-sensitive, relapsed ovarian cancer patients treated with Lynparza (olaparib) tablets (300mg twice daily) compared with placebo in the maintenance setting. The trial met its primary endpoint of investigator assessed PFS (HR 0.30; 95% CI 0.22 to 0.41; P<0.0001; median 19.1 months vs 5.5 months).
PARP inhibitors act in a counter-intuitive manner – by blocking PARP in the context of mutated BRCA1, the cell becomes overwhelmed with double strand breaks, leading to crisis and cell death. BRCA1 mutations, alone, predispose the cell to the accumulation of mutations in protooncogenes and tumor suppressor genes – a few double strand breaks are tumorigenic, whereas a massive number of double strand breaks, as occurs in the context of PARP inhibition, leads to apoptosis.
The use of PARP inhibitors for breast cancer makes great sense, However, in a Phase 3 trial of velparib, an experimental PARP inhibitor, failed to achieve better rates of complete pathogenic response in patients with triple negative breast cancer (TNBC – lack of HER-2, estrogen, and progesterone receptor up-regulation) versus chemotherapy, alone.
At the ASCO conference last week, AstraZeneca presented data on the use of olaparib in 302 patients with BRCA1,2 mutated breast cancer from its OlympiAD trial that compares olaparib against physician’s choice of chemotherapy (capecitabine 2500 mg/m2 d1-14 q 21, or vinorelbine 30 mg/m2 d1,8 q 21, or eribulin 1.4 mg/m2 d1,8 q 21):
OlympiAD Inclusion Criteria:
- Germline mutation in BRCA1 or BRCA2 that is predicted to be deleterious or suspected deleterious.
- Histologically or cytologically confirmed breast cancer with evidence of metastatic disease.
- Prior therapy with an anthracycline and a taxane in either an adjuvant or metastatic setting.
- Prior platinum allowed as long as no breast cancer progression occurred on treatment or if given in adjuvant/neoadjuvant setting at least 12 months from last dose to study entry elapsed.
- ER/PR breast cancer positive patients must have received and progressed on at least one endocrine therapy (adjuvant or metastatic), or have disease that the treating physician believes to be inappropriate for endocrine therapy.
- ECOG performance status 0-1.
- Adequate bone marrow, kidney and liver function.
OlympiAD Exclusion Criteria:
- Prior treatment with PARP inhibitor.
- Patients with HER2 positive disease.
- More than 2 prior lines of chemotherapy for metastatic breast cancer.
- Untreated and/or uncontrolled brain metastases.
Results were quite impressive – this was the first study that demonstrated PARP inhibition is effective in breast cancer:
- About 60% of patients saw their tumors shrink, a hair more than double the 29% objective response rate seen in those patients on chemotherapy.
- Lynparza showed efficacy in patients with TNBC, which is more difficult to treat. AbbVie, which is developing its own PARP inhibitor called veliparib, recentlyannounced a study specifically geared to look at veliparib’s activity in triple negative breast cancer failed to show a benefit when added to chemo.
- Additionally, treatment with Lynparza improved the time to second progression or death compared to chemo,suggesting patients who relapsed after Lynparza experienced a less aggressive return of their cancers.
Astrazeneca is studying olaparib with many combinations, including a study in TNBC with PD-L1 inhibitor durvalumab and CTLA-4 inhibitor tremelimumab.
Cancer vaccine + PD-L1 inhibitor, avelumab (Bavencio), for breast cancer
PD-L1 inhibitor, avelumab (Bavencio – Merck KGaA and Pfizer) will be combined with EpiThany’s EP-101 STEMVAC in patients with breast cancer. Avelumab was recently approved for Merckel cell carcinoma. STEMVAC is a poly-epitope DNA plasmid vaccine in the midst of a phase 1 trial of the vaccine in patients with Stage III or IV patients with breast cancer who have no evidence of disease (NED) or stable bone disease, only. The goals of the study are: Continue reading
CDK4/6 Inhibitors – Excellent Results Support Paradigm Shift in HR+/HER2- Breast Cancer
We have previously written about CDK4/6 inhibitor, palbociclib (Ibrance) for the treatment of patients with hormone receptor positive (HR+) Her-2 negative (HER2-) disease. In a randomized study of 165 patients who had not been treated previously, palbociclib plus letrozole (a nonsteroidal competitive inhibitor of the aromatase enzyme system that inhibits the conversion of androgens to estrogens) was superior to letrozole, alone: Continue reading

Celldex’s ADC Glembatumumab-vedotin for Triple Negative Breast Cancer
Antibody drug conjugate Glembatumumab vedotin (GV) is in late phase 2 trials for patients with triple negative breast cancer (TNBC) – those whose tumors do not express estrogen, progesterone, or HER-2. Approximately 15% of breast cancer patients have TNBC; it is an important area of research for both researchers and clinicians alike because: Continue reading

Biomarkers for Wnt activation Predict For HER-2(-) Breast Cancer and NSCLC Response to Vantictumab
OncoMed is developing several compounds that target Wnt and Notch pathways, which are important in cancer and cancer stem cell maintenance, survival, and proliferation. Biomarkers for response of Her-2-negative breast cancer and non-small cell lung cancer (NSCLC) following treatment with Vantictumab, anti-Wnt monoclonal antibody, have been developed. Continue reading

“Reprogramming” Cancer Cells?
Question: How do you turn aggressive breast, lung, and bladder cancer cells back into benign cells?
Answer: microRNAi’s. Continue reading

Ibrance plus Faslodex Improves Outcomes in HR+ Breast Cancer
In a Phase 3 Paloma-III study of Pfizer’s CDK4/6 inhibitor (Ibrance – palbociclib) and an estrogen receptor antagonist (Faslodex – fulvestrant), patients receiving the combination had significantly prolonged progression-free survival than women with breast cancer who received Faslodex, alone. Continue reading

Entinostat Doesn’t Just Restore Sensitivity to Aromatase Inhibitors
Entinostat is currently in a Phase III trial in combination with Aromasin (exemestane – aromatase inhibitor that blocks the synthesis of estrogen) in patients with metastatic breast cancer who have recurred following treatment with an aromatase inhibitor. Continue reading