PD-L1 inhibitor, avelumab (Bavencio – Merck KGaA and Pfizer) will be combined with EpiThany’s EP-101 STEMVAC in patients with breast cancer. Avelumab was recently approved for Merckel cell carcinoma. STEMVAC is a poly-epitope DNA plasmid vaccine in the midst of a phase 1 trial of the vaccine in patients with Stage III or IV patients with breast cancer who have no evidence of disease (NED) or stable bone disease, only. The goals of the study are:
PRIMARY OBJECTIVES:
- To determine the safety of intradermal administration of up to 3 escalating doses of STEMVAC (CD105/Yb-1/SOX2 CDH3/MDM2-polyepitope plasmid DNA vaccine) in patients with HER2-negative advanced stage breast cancer.
- To determine the most immunogenic dose of STEMVAC in patients with HER2-negative advanced stage breast cancer.
SECONDARY OBJECTIVES:
- To determine whether a STEMVAC T helper 1 cells (Th1) polyepitope plasmid based vaccine elicits a persistent memory T cell response and whether immunity can be further enhanced/maintained by two additional STEMVAC vaccines (boosters) given 3 and 9 months after the priming regimen.
- To evaluate whether STEMVAC vaccination modulates T regulatory cells and myeloid-derived suppressor cells (MDSC).
STEMVAC is administered intradermally with GM-CSF (granulocyte-macrophage colony stimulating factor) every day for 28 days with booster vaccination at 6 and 12 months.
The phase 2 study will include 84 patients receiving avelumab and standard of care chemotherapy will be randomized to undergo vaccination with STEMVAC or placebo.
Rationale for combining approaches
Only 20% of patients with breast cancer have tumor-infiltrating lymphocytes (TILs), a prerequisite for effective checkpoint inhibition therapy. Immune checkpoints inhibit the action of TILs – if they are not present in the tumor microenvironment, the effectiveness of checkpoint inhibition therapy is greatly diminished. TILs are heterogenous, both T1 (cytolytic to cancer cells) and Th2 (suppress the anticancer immune responses – tolerant to cancer antigens). Therefore, inducing Th1 TILs in the microenvironment of breast cancer is critical for effective checkpoint inhibition therapy.
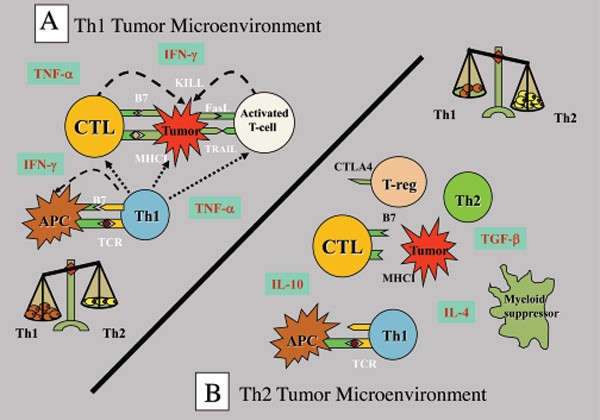
Figure 1. Th1 and Th2 responses in cancer. A Th2 response to cancer is a form of autoimmune disease that protects cancer from cytolytic destruction. http://www.connersclinic.com/cancer-as-an-autoimmune-disease/
STEMVAC (CD105/Yb-1/SOX2 CDH3/MDM2) induces Th1 responses – it consists of DNA that codes for Th1 epitopes of five antigens that are commonly over-expressed in breast cancer:
A plasmid DNA vaccine containing the mammalian expression vector pUMVC3 (pNGVL3) encoding epitopes of CD105 (Endoglin), Y-box binding protein 1 (Yb-1), SRY-box 2 (SOX2), cadherin 3 (CDH3), and murine double minute 2 (MDM2) proteins, with potential immunomodulating and antineoplastic activities. Upon intradermal administration of pUMVC3-CD105/Yb-1/SOX2/CDH3/MDM2-epitopes plasmid DNA vaccine, the plasmid transfects cells and the peptides are expressed. This generates a specific memory Th1 (T-helper) cell immune response, stimulates secretion of cytokines by the T cells and leads to a cytotoxic T-lymphocyte (CTL) response against CD105/Yb-1/SOX2/CDH3/MDM2-expressing tumor cells. CD105/Yb-1/SOX2/CDH3/MDM2 proteins are highly immunogenic tumor associated antigens that are overexpressed in breast cancer. Additionally, these antigens are associated with breast cancer stem cells and with epithelial to mesenchymal transformation (EMT).
The five cancer antigens to which STEMVAC enhances a Th1 immune response have important roles in the progression and prognosis of breast cancer:
- Endoglin expression in metastatic breast cancer enhances invasiveness by increasing the activity of invadopodia and the amount of matrix metalloproteinase expression.
- Y-box protein 1 binds to DNA and RNA and is a regulator of transcription and translation; over-expression of this protein is correlated with reduced overall survival and hormone receptor negativity, worse histologic and nuclear grade, high tumor stage, lymphovascular invasion and high Ki67(proliferation marker).
- SRY-box 2 [sex-determining region Y (SRY)-box binding protein-2 (Sox2)] is a transcription factor that is integral to maintaining pluripotent stem cell state in embryonic stem cells (ESCs) and induced pluripotent stem cells.
- Cadherin 3 (P-cadherin) is an adhesion protein – aberrant expression is seen in 20-40% of invasive breast cancers and is associated with poor clinical outcome (Figure 2). It is an enhancer of migration and invasion.
- MDM2 (murine double minute protein 2) inhibits p53 functioning. Patients with MDM2-positive breast cancers have a worse prognosis: disease specific survival in patients expressing MDM2 is lower than in those whose tumors do not express MDM2.
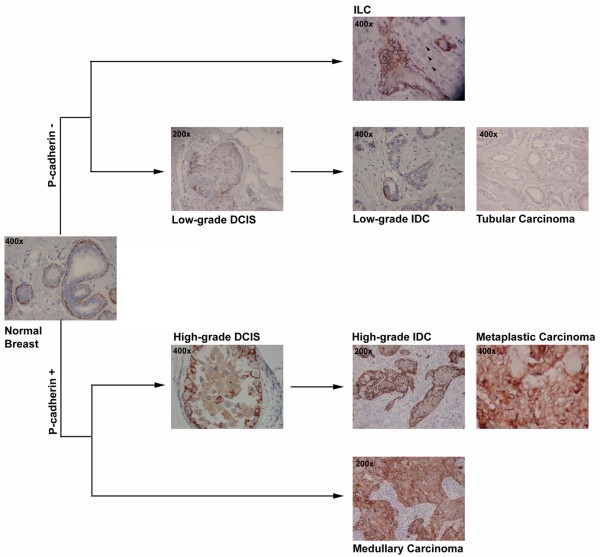
Figure 2. Expression of P-cadherin in breast tissue. Schematic representation of P-cadherin positive and negative breast cancer histological types. In normal breast, P-cadherin is only expressed by myoepithelial cells and not by luminal epithelial cells. In the low-grade arm of breast carcinomas, the majority of lesions are negative for P-cadherin expression, such as invasive lobular carcinomas (ILC), tubular carcinomas, and well differentiated ductal carcinoma in situ (DCIS) and invasive ductal carcinomas (IDC). In the other arm, high-grade lesions are frequently positive for this cadherin, including the medullary carcinomas, a subset of poor-differentiated DCIS and IDC, and the metaplastic carcinomas, which represent a clear subtype of basal-like carcinomas. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2242663/figure/F2/
