A course of treatment with checkpoint inhibitors Yervoy (ipilimumab) and Opdivo (nivolumab) for patients with unresectable or metastatic melanoma is every 3 weeks for a total of four doses. Almost forty percent of patients receiving this combined regimen discontinue treatment because of immune-related adverse events. Toxicities that contribute to early discontinuation of the 4-dose combined checkpoint regimen include diarrhea, colitis, elevated liver enzymes. Systemic steroids are the most frequently used immunosuppressive therapy for the management of adverse events on checkpoint inhibitors. Limiting the course of therapy and administering immunosuppressive therapy to manage toxicities on combined checkpoint inhibition, however, could abrogate the desired anti-cancer effects.
Does discontinuation of combined checkpoint inhibition therapy negatively affect efficacy?
Researchers at Sloan Kettering performed a pooled analysis of patients participating in the Phase II CheckMate 069 and Phase III CheckMate 067 trials in order to determine the impact of early discontinuation and treatment efficacy. Patients were included in the efficacy analysis if they had received at least one dose of nivolumab and ipilimumab as combination therapy and discontinued treatment because of adverse events during the the induction phase of treatment (n = 96) or did not discontinue treatment because of adverse events (n = 233).
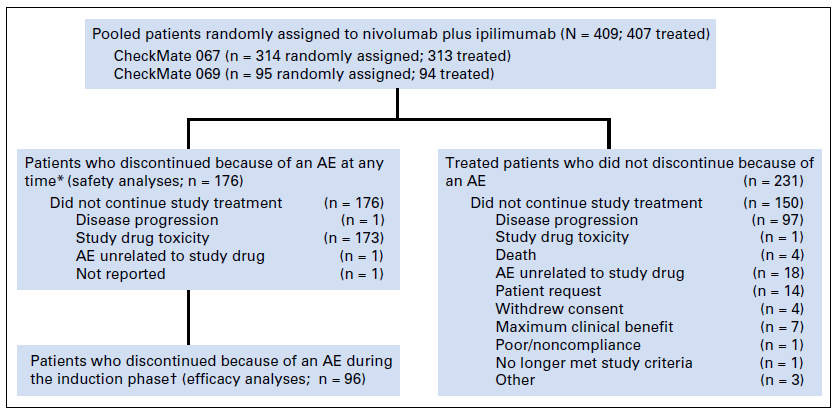
Figure 1. Patient populations included in this pooled analysis. (*) Includes patients who discontinued and had a treatment-related adverse event (AE) at any time. (†) For patients
who had taken at least one dose of nivolumab monotherapy, the induction phase was defined as the time between the first dose of nivolumab plus ipilimumab up to a day before
the first nivolumab monotherapy dose. For patients who discontinued before receiving
nivolumab monotherapy, the induction phase was defined as the time between the first and
the last dose of combination treatment. http://ascopubs.org/doi/pdf/10.1200/JCO.2017.73.2289
There were no statistically significant differences between the two groups with respect to treatment effects (Table 1). Median progression-free survival was 8.4 months (95% CI, 5.8 to 16.7 months) and 10.8 months (95% CI, 5.9 to 23.0 months) for those who discontinued because of adverse events and those who did not discontinue (p = 0.966). Overall survival rates at 18 months were 67% for those who discontinued early due to adverse events versus 62% for those who did not discontinue – hazard ratio for overall survival 0.79, p = 0.2344.
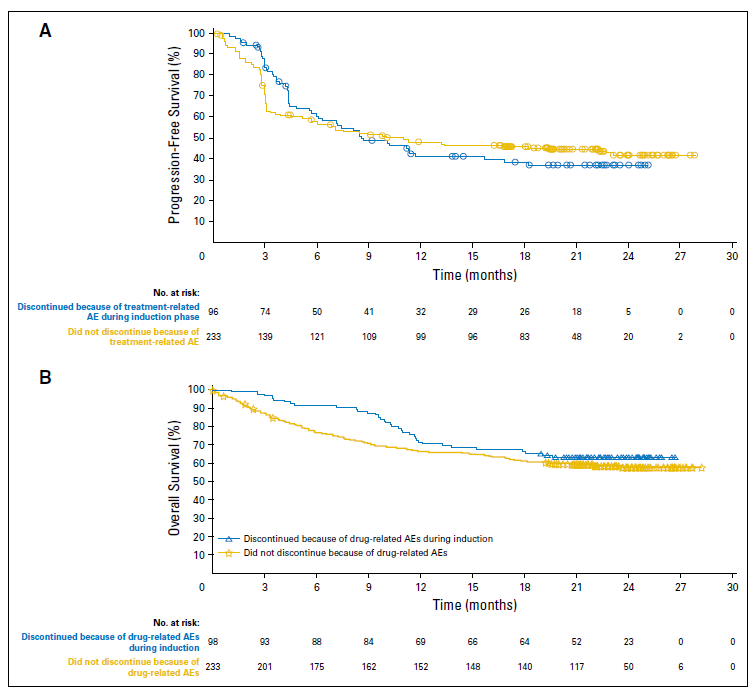
Figure 2. (A) Progression-free survival and (B) overall survival for patients who discontinued treatment because of adverse events (AEs) during the induction phase and for
patients who did not discontinue because of AEs. Differences between the two subgroups were not statistically significant for either progression-free survival or overall
survival. http://ascopubs.org/doi/pdf/10.1200/JCO.2017.73.2289
With respect to safety, 156 of 407 patients (38%) discontinued treatment because of adverse events. Colitis was the most frequently reported treatment-related adverse event; it led to discontinuation in 10% of patients, followed by elevated liver enzymes (9%). Colitis was also the main serious adverse event leading to hospitalization, occurring in 21% of the patients who discontinued due to an adverse event and in 2.6% of patients who did not discontinue.
Systemic corticosteroids were used in 91% of patients who discontinued because of an adverse event and in 55% of patients who did not discontinue treatment. Infliximab (anti-TNF antibody) was used in 10% of patients who discontinued, compared to 1% who did not discontinue treatment. This difference is attributable to the higher incidence of colitis in patients discontinuing treatment due to adverse events as compared with those who did not discontinue therapy.
Implications of the analysis
- “The median reduction in tumor burden was 51.4% across both subgroups, which is in keeping with the rapid tumor response kinetics seen with ipilimumab and nivolumab combination treatment. These data reassure patients and clinicians that the development of early and severe toxicity that necessitates treatment cessation before completion of induction treatment does not seem to reduce the chance of response and might be associated with an increased probability of response.”
- “In summary, several key messages have arisen from this work. The response rate, depth of response, and OS do not seem to be diminished for patients who discontinue treatment as a result of irAEs (immune-related adverse events) at induction. In addition, the use of immunomodulatory agents to manage checkpoint inhibitor–related toxicities does not seem to hinder responses or durability of response in the majority of patients who discontinue treatment because of irAEs at induction. The merits, liability, and optimal duration of ongoing anti-PD-1 therapy after discontinuing induction therapy because of irAEs will require prospective evaluation. Finally, the predictive utility of early-onset severe irAEs on efficacy outcomes needs additional study in larger cohorts

