The results of CheckMate 214 demonstrated that combination checkpoint immunotherapy with nivolumab (Opdivo; anti-PD-1 monoclonal antibody) and ipilimumab (Yervoy; anti-CTLA-4 monoclonal antibody), is superior to sunitinib (Sutent; multikinase inhibitor) in the treatment of patients with newly diagnosed renal cell carcinoma (RCC). Interestingly, prior to sunitinib, another immunotherapeutic approach – interferon-alpha (IFN-α) – was the front-line treatment of choice for renal cell carcinoma, which, like melanoma, is very immune-responsive.
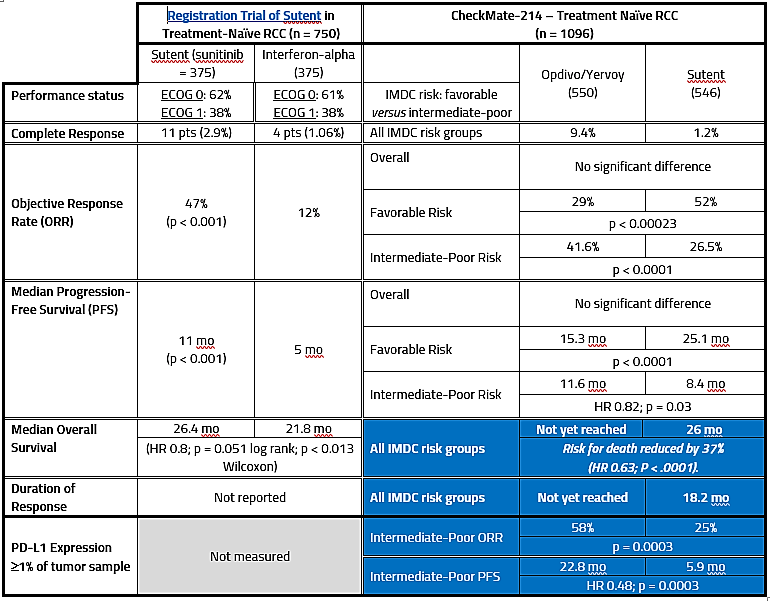
In the Phase 3 study that established sunitinib as the front-line treatment of choice, 750 treatment-naïve patients (ECOG performance status 0 or 1; 62% and 38%, respectively) were randomized 1:1 to sunitinib or IFN-α. Sunitinib was superior to IFN-α in terms of progression-free interval [PFS; 47.3 months vs 22.0 months; Hazard Ratio (HR) 0.14; p< 0.001], and objective response rate (ORR; 47% vs 12%; p < 0.001). Median overall survival was 114.6 weeks for sunitinib and 94.9 weeks for IFN-α. Overall survival was 26.4 months for sunitinib versis 21.8 months for IFN-α (HR 0.821; p = 0.051 log rank and p < 0.013 Wilcoxon).
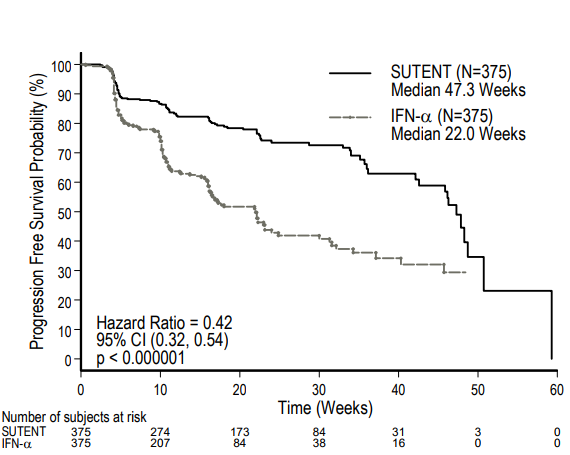
Figure 1. Sutent versus IFN-α in treatment-naïve renal cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021938s13s17s18lbl.pdf

Figure 2. Overall survival of Sutent versus IFN-α. http://ascopubs.org/doi/abs/10.1200/JCO.2008.20.1293?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed&
CheckMate-214 Results
The study included patients with advanced (Stage IV – not amenable to surgery or radiation therapy) histologically-confirmed renal cell carcinoma who had not received prior systemic chemotherapy (except for neo-adjuvant therapy for completely resectable RCC). Patients were excluded if metastases to the central nervous system were present or if the neo-adjuvat therapy included anti-VEGF treatment. The co-primary endpoints of CheckMate-214 were ORR, PFS per independent committee and overall survival (OS) in the cohort of patients at intermediate or poor-risk. Efficacy was also evaluated according to IMDC (International Metastatic RCC Database Consortium) risk group and baseline tumor PD-L1 expression. Checkmate-214 was initiated in October 2014.
Top-line results indicated that Opdivo/Yervoy (n = 550 patients) resulted in a 41.66% objective response rate, vs 26.5% (p < 0.0001) for Sutent (n = 546 patients) – 9.4% achieved complete response on Opdivo/Yervoy versus 1.2% for Sutent:
Median duration of response was not reached for the combination of Opdivo and Yervoy and was 18.17 months for sunitinib. The median PFS was 11.56 months (95% CI 8.71 – 15.51) for the Opdivo and Yervoy combination versus 8.38 months (95% CI 7.03-10.81) for sunitinib.
The median OS was 26 months for sunitinib and has not yet been reached for the combination. With a minimum follow-up of 17.5 months, the combination reduced the risk for death by 37% (hazard ratio [HR], 0.63; P < .0001).
Patients with the immunotherapy combination had fewer grade 3-5 adverse events than those who received sunitinib (45% vs 63%), and they reported better quality of life and better control of their symptoms. Discontinuation due to an adverse event (AE) was reported for 22% of combination patients and 12% of sunitinib patients. Of the 159 deaths that occurred in the combination cohort, seven (1%) were considered drug-related, whereas 4 (1%) of the 202 deaths occurring in the sunitinib cohort were considered drug related.
Looking deeper at PD-L1 expression and IMDC risk group:
Both the ORR per independent committee and PFS significantly favored nivolumab plus ipilimumab over sunitinib in intermediate/poor risk patients having baseline PD-L1 expression ≥1% where the ORR was 58% versus 25%, and median PFS was 22.8 (95% CI 9.4, NR) months versus 5.9 (95% CI 4.4, 7,1) months, respectively, HR 0.48 (95% CI 0.28, 0.82; p = 0.0003).
The investigators found that baseline tumor PD-L1 expression was lower in the cohort of patients at favorable risk where 11% of patients on combination had PD-L1 levels ≥1% versus12% of patients on sunitinib compared to 26% versus 29% of patients at intermediate or poor risk in the respective treatment arms.
In patients at favorable risk, both the ORR and PFS were higher with sunitinib over combination; in this cohort, ORR was 29% with nivolumab/ipilimumab versus 52% with sunitinib (p = 0.0002) and median PFS was 15.3 (95% CI 9.7, 20.3) months versus 25.1 (95% CI 20.9, NR) months, respectively, HR 2.17 (95% CI 1.46, 3.22; p < 0.0001).
In the overall composite of patients at any risk, no significant difference between treatments was demonstrated in ORR (p = 0.0191) or PFS (p = 0.819).
Conclusion
The results support the use of Opdivo/Yervoy combination front-line in patients with intermediate/poor risk metastatic disease with PD-L1 expression ≥ 1%. However, Sutent remains the treatment of choice for patients with favorable risk (see Table 1).
The results of CheckMate-214 were very well received at ESMO (European Society of Medical Oncology):
Until now, nothing has beaten sunitinib in a randomized phase 3 clinical trial, and it was “brave to choose it as a comparator,” said Manuela Schmidinger, MD, from the Medical University of Vienna, Austria. She pointed out that this is the first phase 3 study to show a statistically significant OS benefit.
This new study changes the paradigm, and it establishes a new standard of care for the first-line treatment of advanced RCC, she commented. Dr Schmindinger was not involved in the study and acted as discussant after it was presented here in a presidential session at the European Society of Medical Oncology (ESMO) 2017 Congress.
Several others agreed with her. This is practice changing, commented Markus Joerger, MD, PhD, assistant professor of medical oncology and clinical pharmacology, St. Gallen Cancer Center, Switzerland, who chaired the ESMO press briefing at which the study was highlighted.

Hi. I am not aware of the results with Cabosun. As far as Checkmate 214, I believe that the initial top-line data were not received well. It was only after the data at ESMO were revealed (effectiveness in high risk patients) physicians treating the disease heralded it as a significant contribution. If you would like to contribute a guest post on something, please let me know.
“Until now no one has beaten Sunitinib in a randomized phase 3 clinical trial” . Why do you leave out the exciting results of the Cabosun trial. Yes it was only a ph2 trial, but it handily beat Sunitinib according to the IRR. If you were unaware of this trial Dr. Pal and Dr. Choueiri are very familiar with the results, you could check up on their articles
What I would love to see is a ph3 trial comparing Cabo to Opdivo or Cabo w/ Nivo. It looks like Checkmate 9ER is the closest we will get.
I would also mention the Checkmate 214 results were not well received by all. Important information was left out and I think curious people should wonder why. A couple of well written article were posted about this on EP Vantage.