Immune checkpoint inhibitors are simply cancer wonder drugs about which we are learning more each day. Because they don’t work optimally in many patients and some even hyper-progress, the goal is to determine ways to expand their effectiveness to more patients. As such, the number of clinical studies with checkpoints and checkpoint combinations continues to grow.
Immune checkpoint inhibitors act by blocking the abrogating phase of the immune response that is necessary to prevent autoimmune disease – by prolonging the immune response against cancer, a more robust and prolonged immune response, which is required for effective cancer therapy, is achieved with checkpoint therapy.

Figure 1. Immune checkpoint blockade. Inhibitory receptors such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) expressed on tumor-specific T cells lead to compromised activation and suppressed effector functions such as proliferation, cytokine secretion, and tumor cell lysis. Modulating these receptors using monoclonal antibodies, an approach termed “immune checkpoint blockade,” has gained momentum as a new approach in cancer immunotherapy. This treatment concept was first introduced in patients with advanced melanoma: in this patient population, the anti-CTLA-4 antibody ipilimumab was the first drug ever to show improved overall survival in phase III trials. Antibodies directed against PD-1 and its ligand, PD-L1, have shown much promise in the treatment of melanoma, renal cell cancer, non-small cell lung cancer, and other tumors, as evident by encouraging rates and durability of tumor responses. Because of the successes with immune checkpoint inhibitors in cancer immunotherapy, many new agents and strategies, including combination approaches, are being developed at a fast pace. http://www.onclive.com/publications/contemporary-oncology/2014/february-2014/immune-checkpoint-blockade-in-cancer-inhibiting-ctla-4-and-pd-1pd-l1-with-monoclonal-antibodies
Recently, four large clinical trials with checkpoints inhibitors yielded disappointing results:
- BMS’ combination of ipilimumab (Yervoy – CTLA-4 inhibitor) and nivolumab (Opdivo) failed to show an improvement in progression-free survival versus sunitinib (Sutent – kinase inhibitor) in patients with previously untreated advanced or metastatic renal cell carcinoma:
- The CheckMate -214 trial randomised patients with previously untreated advanced or metastatic renal cell carcinoma to receive Opdivo plus Yervoy, or Sutent alone. The main goals of the study are PFS, objective response rate and overall survival, with Bristol-Myers Squibb noting that “the majority of alpha was allocated” to overall survival. According to Bristol-Myers Squibb, median PFS was 11.56 months for the Opdivo and Yervoy combination compared to 8.38 months for Sutent. Meanwhile, the drugmaker noted that Opdivo plus Yervoy achieved a 41.6 percent objective response rate versus 26.5 percent for Sutent, meeting one of the trial’s co-primary endpoints. Data showed that the median duration of response was not reached for the combination of Opdivo and Yervoy, and was 18.17 months for Pfizer’s drug. Bristol-Myers Squibb added that the study will continue as planned to allow the third co-primary endpoint of overall survival to mature.
- AstraZeneca’s MYSTIC trial of durvalumab (Imfinzi – PD-L1 checkpoint inhibitor) and tremelimumab, a CTLA-4 (cytotoxic T lymphocyte-associated protein 4) inhibitor in patients with front-line non-small cell lung cancer (NSCLC). The study randomized patients to standard of care (platinum-based chemotherapy), Imflinzi, or the PD-L1/CTLA-4 combination. Although progression-free survival (PFS) was not positive, the study’s primary outcome is overall survival (OS) and data on this endpoint will be available in the first half of 2018:
- The phase 3 test was seeking to boost progression-free survival (PFS) in advanced, first-line non-small cell lung cancer patients using either its PD-L1 checkpoint inhibitor Imfinzi (durvalumab) as a monotherapy, or Imfinzi in combo with CTLA-4 drug tremelimumab. The medications were pitted against platinum-based standard-of-care, but after much speculation the British-based drugmaker said it had failed to improve PFS when compared to chemo in patients whose tumors express PD-L1 on 25% or more of their cancer cells. AZ isn’t giving up completely, however, and said: “The trial will continue to assess two additional primary endpoints of overall survival (OS) for Imfinzi monotherapy and OS for the Imfinzi plus tremelimumab combination.”
- Merck’s Keynote-040 trial of Keytruda (pembrolizumab) versus standard treatment (methotrexate, docetaxel or cetuximab) for the treatment of recurrent or metastatic head and neck squamous cell cancer (HNSCC) did not did not meet its pre-specified primary endpoint of OS (HR, 0.82 [95% CI, 0.67-1.01]; p = 0.03 [one-sided]). However, another study in this claim, Keynote-048, is still ongoing:
- KEYNOTE-040 is a randomized, multi-center, pivotal phase 3 study (ClinicalTrials.gov, NCT02252042) investigating KEYTRUDA as a monotherapy versus standard treatment (methotrexate, docetaxel or cetuximab) for the treatment of recurrent or metastatic HNSCC. The primary endpoint is OS; secondary endpoints include progression-free survival (PFS) and overall response rate (ORR). The study, which opened inNovember 2014, enrolled 495 patients to receive KEYTRUDA (200 mg fixed dose every three weeks) or investigator-choice chemotherapy (methotrexate [40 mg/m2 on Days 1, 8, and 15 of each 3-week cycle], docetaxel [75 mg/m2 on Day 1 of each 3-week cycle], or cetuximab [400 mg/m2 loading dose on Day 1 and 250 mg/m2 IV on Days 8 and 15 of Cycle 1], followed by cetuximab [250 mg/m2 on Days 1, 8, and 15 of each subsequent 3-week cycle]). Patients enrolled in the study had been previously treated with 1-2 platinum-containing systemic regimens.
- Roche/Genentech’s IMvigor211 study of Tecentriq (atezolizumab) failed to meet its primary endpoint of overall survival for patients with locally advanced or metastatic urothelial cancer. The study enrolled patients who had progressed following platinum-based chemotherapy and randomized treatment to Keytruda or chemotherapy:
- The results observed in people treated with TECENTRIQ in IMvigor211 were generally consistent with those observed in a similar group of people in the Phase II IMvigor210 study. The IMvigor211 data will be further examined in an effort to better understand these results, including the initial observation that the chemotherapy arm results were better than study design assumptions. Full data from IMvigor211 will be presented later this year.
Considering the positive trends in the endpoints achieved in the trials of PD-1/PD-L1 + CTLA-4 combinations (renal cancer and NSCLC discussed in #1 and #2, above), the overall survival analyses may, indeed, prove that combined checkpoint therapy is beneficial. Whether or not this is the case, the field is undeterred. Undaunted by the setbacks, and as an indication of the opportunity that checkpoint inhibitor therapy represents, AstraZeneca and Merck signed a collaboration to investigate combinations of drugs, including Lynparza (olaparib, a PARP inhibitor) and Keytruda (PD-1), Imflizi (PD-l1), tremelimumab (CTLA-4), and selumetinib (MEK inhibitor).
Lynparza is approved for ovarian cancer patients with BRCA mutations, however, in a phase 3 study versus standard-of-care chemotherapy in patients with HER2-negative breast cancer and BRCA1 or BRCA2 mutations, Lynparza lowered the risk of disease worsening or death by 42%.
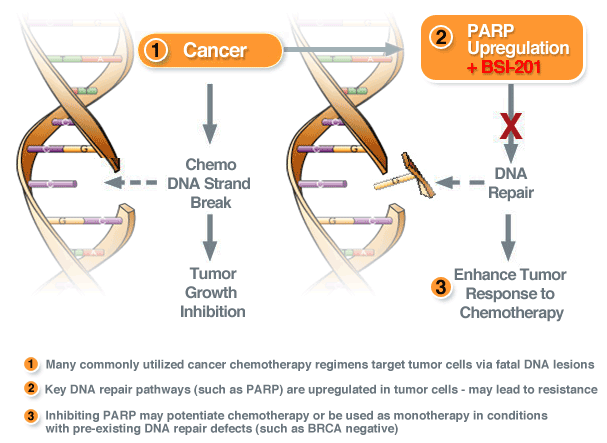
Figure 2. PARP inhibition. PARP Inhibitors can be used for ovarian, breast and prostate cancer and generally target mutations in the BRCA1, BRCA2 and inhibitor azd2281. These drugs represent a new way to treat BRCA-associated cancers, with the parp inhibition having the potential to overwhelm cancer cells and whilst they are not a cure for cancer the inhibition for the body to produce parp, leads to the BRCA cancer cells unable to be repaired as they grow: ultimately leading to the cancer inhibition and not being able to grow.
The reason this drug is such a promising target is that it has demonstrated significant anti-tumor effects in several types of cancer and parp inhibition does not appear to be critical for normal non-cancerous cells, thus parp inhibitors have the potential to impair tumor growth without damaging the normal cells, and therefore causing less side effects for those that receive the inhibitor cancer treatment. If parp trials are successful this may be one of the most famous anti-cancer drugs. http://www.parp-inhibitors.com/
And, Keytruda has shown benefit in patients with triple negative breast cancer (TNBC – lack of over-expression of estrogen, progesterone, and HER-2) in a phase 2 trial:
The trial included two groups of patients with advanced breast cancer that had spread to other locations. The first group was composed of 170 patients who had received extensive earlier treatment. Keytruda is a checkpoint-blocking drug, and patients in the first group were included regardless of whether the checkpoint molecule PD-L1 was present in the tumor. The second group had not received earlier treatment and had tumors expressing PD-L1. Among pre-treated patients, Keytruda shrank tumors by more than 30 percent in eight (5 percent) of the women, and stabilized disease in 35 women (21 percent) of the group. All eight patients who saw their tumors shrink lived for at least another year. In comparison, the patients who did not experience tumor regression had lower survival rates.
In the group that received Keytruda as their first treatment, 12 of 52, or 23 percent, saw tumors shrink by more than 30 percent. The treatment also stabilized disease in another 17 percent. The study is the first triple negative breast cancer immunotherapy trial to date, and also is the largest study of immunotherapy in this cancer form.
I am anxious to see the results of combination studies of Lynparza and Keytruda in patients with breast cancer.
