Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading

Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading
Sellas reversed merged into Galena, a peptide vaccine company whose lead product, NeuVax, for breast cancer failed. Sellas’ lead product is galinpepimut-S for AML (acute myelogenous leukemia) and mesothelioma, as well as other cancers. Continue reading
Two companies – Biontech, RNA vaccine and Neon Therapeutics, peptide vaccine – reported very positive results of neo-antigen vaccines in patients with refractory metastatic melanoma. Continue reading
Olaparib (Lynparza) is a PARP (poly-ADP ribose polymerase) inhibitor that was approved by the FDA in 2014 for the treatment of patients with advanced ovarian cancer who have mutated BRCA1,2 gene. Recently, the drug showed a 70% reduction in risk of progression in patients with less-advanced disease in the maintenance therapy setting:
The Phase III SOLO-2 trial demonstrated a significant improvement in progression-free survival (PFS) in germline BRCA-mutated (gBRCA), platinum-sensitive, relapsed ovarian cancer patients treated with Lynparza (olaparib) tablets (300mg twice daily) compared with placebo in the maintenance setting. The trial met its primary endpoint of investigator assessed PFS (HR 0.30; 95% CI 0.22 to 0.41; P<0.0001; median 19.1 months vs 5.5 months).
PARP inhibitors act in a counter-intuitive manner – by blocking PARP in the context of mutated BRCA1, the cell becomes overwhelmed with double strand breaks, leading to crisis and cell death. BRCA1 mutations, alone, predispose the cell to the accumulation of mutations in protooncogenes and tumor suppressor genes – a few double strand breaks are tumorigenic, whereas a massive number of double strand breaks, as occurs in the context of PARP inhibition, leads to apoptosis.
The use of PARP inhibitors for breast cancer makes great sense, However, in a Phase 3 trial of velparib, an experimental PARP inhibitor, failed to achieve better rates of complete pathogenic response in patients with triple negative breast cancer (TNBC – lack of HER-2, estrogen, and progesterone receptor up-regulation) versus chemotherapy, alone.
At the ASCO conference last week, AstraZeneca presented data on the use of olaparib in 302 patients with BRCA1,2 mutated breast cancer from its OlympiAD trial that compares olaparib against physician’s choice of chemotherapy (capecitabine 2500 mg/m2 d1-14 q 21, or vinorelbine 30 mg/m2 d1,8 q 21, or eribulin 1.4 mg/m2 d1,8 q 21):
OlympiAD Inclusion Criteria:
OlympiAD Exclusion Criteria:
Results were quite impressive – this was the first study that demonstrated PARP inhibition is effective in breast cancer:
Astrazeneca is studying olaparib with many combinations, including a study in TNBC with PD-L1 inhibitor durvalumab and CTLA-4 inhibitor tremelimumab.
The latest checkpoint inhibitor to be approved is AstraZeneca’s Imfinzi (durvalumab), a monoclonal antibody directed against PD-L1, which is expressed on cancer cells.
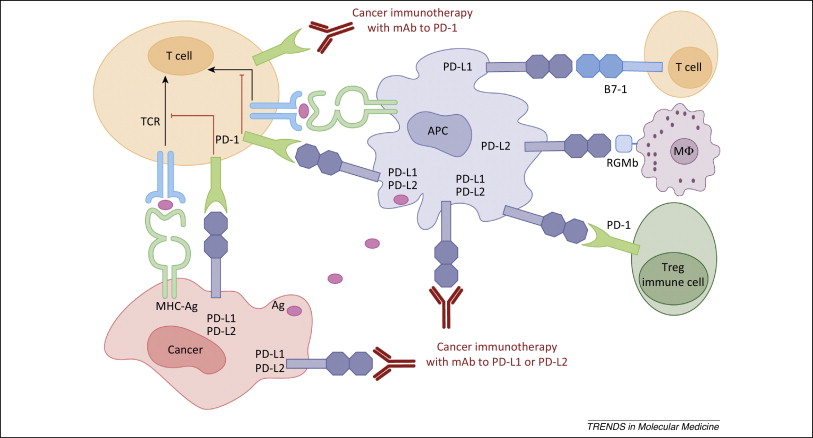
Figure 1. PD-1 / PD-L1 axis. http://www.cell.com/trends/molecular-medicine/references/S1471-4914(14)00183-X
Immune checkpoint-directed therapy is producing unprecedented clinical results in many patients. So much so, that the FDA recently reversed its longstanding policy or approving cancer drugs based on site of origin, to the presence of a biomarker (microsatellite instability (MSI-H) or mismatch-deficient repair (dMDR) as the indication for therapy with pembrolizumab (Ketruda), and PD-1 blocker. Cancers expressing MSI-H or dMDR mutate at a rapid rate, presenting novel epitopes to the immune system, which is readily mobilized against them so that tumor infiltrating T-cells are reliably present. Blocking the PD-1/PD-L1 pathway in this context allows for prolongation of the immune response and better clinical results. Continue reading
Platelets are the second most abundant cellular component of blood. The platelet membrane contains an abundance of receptors to facilitate interactions with subendothelial matrix , other blood cells, and other platelets. The central role of platelets is in hemostasis, however, they also contain copious amounts of cytokines that induce inflammation. Continue reading
Avelumab (Bavencio) is a PD-L1 inhibitor that was approved for the treatment of patients with metastatic Merkel cell carcinoma (MCC). Continue reading
Results with checkpoint inhibitors nivolumab (PD-1, Opdivo), pembrolizumab (PD-1, Keytruda), and atezolizumab (PD-L1, Tecentriq) are impressive. Some patients have experienced incredible and prolonged responses. These drugs are truly modern medical breakthroughs.
Continue reading
Cancer and autoimmune disorders are opposite sides of the same coin – cancer is a result of hypo-active immunity, whereas, autoimmune diseases is the result of hyper-active immunity. This is dramatically illustrated in examining side effects in patients with melanoma who receive checkpoint therapy with ipilimumab (Yervoy), which acts in the early stages of T-cell activation and priming, and nivolumab (Opdivo), which acts in the later stages of T-cell activation in the tumor microenvironment. Continue reading