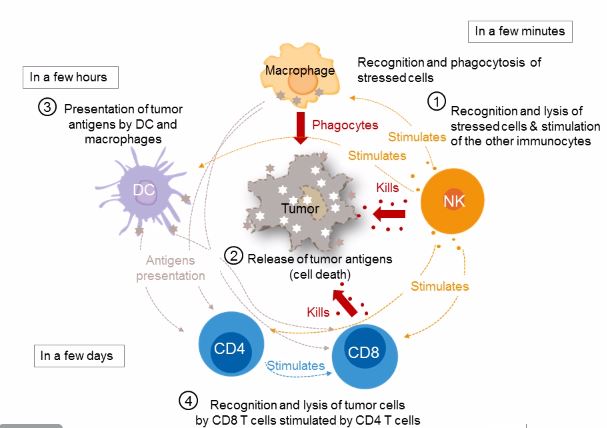
Natural Killer cells and macrophages are essential in the innate immune response to bacterial pathogens. They also provide the essential link to the adaptive immune response by presenting antigens to dendritic cells and by directly stimulating CD8+ cytotoxic T-cells.