On April 29, the FDA Oncology Drug Advisory Committee voted 22-1 to recommend approval of Amgen’s talimogene laherparepvec (T-VEC) for the treatment of patients with advanced melanoma.
T-VEC is an oncolytic herpes simplex virus genetically engineered to replicate only in cancer cells and to express GM-CSF. The Phase III study randomized patients to intra-lesional injection of T-VEC versus GM-CSF alone.
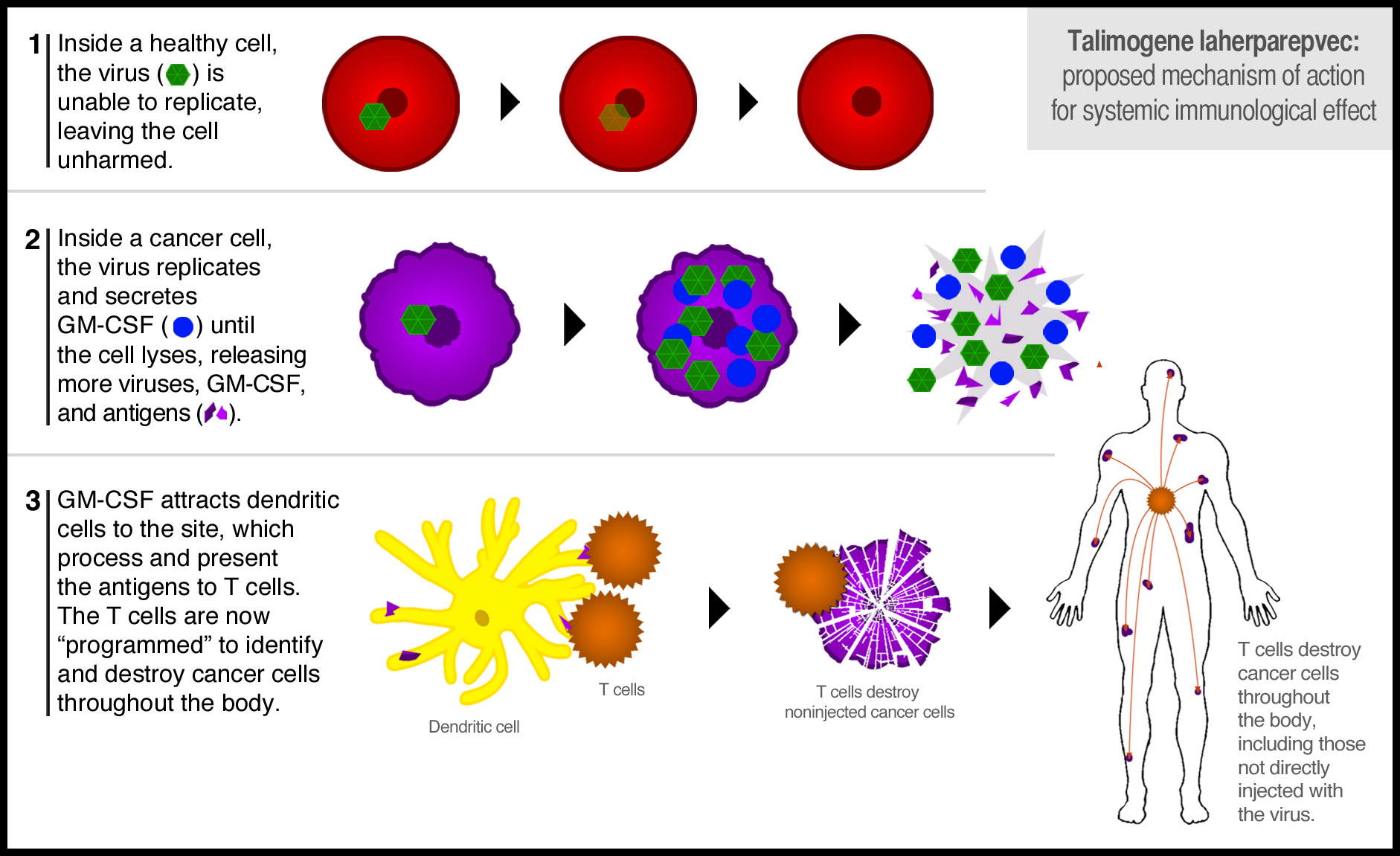
How does T-VEC work? There are two important elements – replication of the virus so that it lyses the host melanoma cells, spilling tumor antigens for the dendritic cells that are recruited by the expression of GM-CSF to present to the immune system to trigger a strong immunologic response.
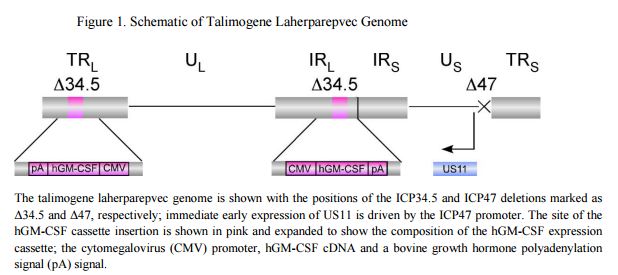
Talimogene laherparepvec was derived from a novel primary HSV-1 isolate (JS1, ECACC Accession Number 01010209) that demonstrates enhanced oncolytic activity towards tumor cells, as compared to the commonly used laboratory strains (e.g., 17syn+) and other primary isolates (Liu et al., 2003). To produce talimogene laherparepvec, the JS1 strain was genetically modified by deleting the virulence genes that code for ICP34.5 and ICP47. Wild type HSV-1 contains two copies of the gene for ICP34.5, and both copies were functionally deleted in talimogene laherparepvec by inserting two copies of human GM-CSF gene sequences. Deletion of the ICP47 gene also resulted in converting the HSV-1 late gene US11 into an immediate early gene, under the ICP47 promoter (Cassady et al., 1998). A schematic of the talimogene laherparepvec genome is shown in Figure 1.
Cell culture studies of ICP34.5 depleted HSV-1 demonstrated that embryonic cells and cancer cells are lysed by infection of this construct, but, differentiated cells are not. Rapidly dividing cells, then, produce a pro’tein that, either alone or in a complex with other cellular and/or viral proteins, can replace ICP34.5 to allow virus replication.
Although the Phase III study did not include combination therapy with a checkpoint inhibitor [anti-PD-1 – Keytruda (pembrolizumab) or Opdivo (nivolumab), or anti-CTLA-4 – Yervoy (ipilimumab)], studies are underway to do just that. The logic behind this is straightforward – T-VEC treatment initiates a robust immune response, and the checkpoint inhibitor ensures that the response is not abrogated. In the Phase III study, patients receiving T-VEC therapy first-line did better than those receiving it as second-line therapy, which supports the combination use with checkpoint inhibitors since the immune response is less likely to have been abrogated in front-line patients. In second-line, where the immune system is more likely to have seen cancer antigens previously (due to cancer cell killing), there is a greater likelihood that the patients were experiencing an abrogating second wave immune response when T-VEC was administered, therefore, diminishing its effect.
The Phase III study results demonstrated the following:
- The study involved 436 patients and T-VEC significantly improved durable response rate (DDR) with 3 percent of T-VEC patients achieving a complete response (CR) or partial response (PR) within the first 12 months of treatment.
- Patients taking T-VEC had a median overall survival of 4.4 months longer than those taking GM-CSF;
- T-VEC patients who experienced a durable response in the first year of treatment had a 95% reduction in the risk of death. Among 2,116 melanoma tumors injected with the drug, 995 completely disappeared. And 212 tumors that weren’t injected also vanished, suggesting the drug may, in fact, be stimulating the immune system to kill cancer on its own, as designed;
- The most common adverse events observed in the T-VEC trial were flu-like symptoms, which seemed to subside after the third course of treatment.


I just searched, myself. I could not find it. This was an early post – I have since added captions with links to the images. Sorry!
Dear Joseph Gulfo,
I also would like the nice image regarding mechanism of action, but I haven´t found the reference you mention. It would be great if you could forward it.
Thank you very much!
You need to reference the article from which I took it – there are links in my piece to it.
I was wondering if I could use your image with the proposed mechanism of action of t-vec in an upcoming presentation that I am giving about the use of intra-lesional therapies in melanoma.