Sitravatinib (MGCD516) is an oral multi-tyrosine kinase inhibitor being developed by Mirati Therapeutics. Last week, the company announced that three of eleven patients with non-small cell lung cancer (NSCLC) with genetic alterations in MET, AXL, RET, TRK, DDR2, KDR, PDGFRA, KIT or CBL who were resistant to checkpoint [anti PD-(L)1 therapy] had confirmed partial responses; because of this, dosing in the 34-patient expansion cohort will proceed. Continue reading
Category Archives: Biology
New Link’s Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment. Continue reading
WP1122 is a 2-deoxy-D-glucose prodrug in pre-clinical development for glioblastoma
The Warburg Effect is a universal feature of cancer; it describes the phenomenon whereby cancer cells preferentially use glucose for anaerobic glycolysis, as opposed to aerobic respiration via the Krebs Cycle. In order to meet the increased energy demands using a much less efficient process for ATP production, cancer cells take-up 20-times more glucose than wild-type cells. Continue reading
OLIG2 inhibitor for glioblastoma
OLIG2 (Oligodendrocyte transcription factor-2) is a transcription factor that is expressed in the pMN domain of the ventral ventricular zone in the embryonic spinal cord. Along with OLIG1, it is responsible for the development of motoneurons and oligodendrocytes. Astrocytes and ependymal cells also originate from the pMN domain. Continue reading
MDM2 and MDMX inhibitor restores p53 functioning in cancers with wild-type p53
P53 is a tumor suppressor gene that pauses cell division to allow for repair of gene damage, and triggers apoptosis if the damage is not reparable. Loss of p53 is a critical step in the evolution of cancer. Most frequently, p53 is mutated at its DNA binding domain; since p53 is a transcription factor, a diminished ability to bind to DNA significantly disrupts its functioning. Continue reading
Lactate is another energy source for cancer cells
We have written previously about the Warburg Effect, the observation that cancer cells “bypass normal cellular respiration, that is, glucose converted to pyruvate through glycolysis, and the sequential oxidation of pyruvate through the Krebs Cycle in the mitochondria. Instead, tumor cells divert pyruvate to lactate dehydrogenase (LDH), which reduces pyruvate into lactate.” Continue reading
Blocking Protein-Protein Interactions in Cancer
The last twenty years has been an unprecedented time in biology – in sequencing the genome and studying the functions of proteins, as well as in unraveling signal transduction pathways, the fundamental biology of normal and diseased cells has been elucidated to a great extent. Although many druggable targets have been identified, it has largely been impossible to target protein-protein interactions (PPI) in drug development. In fact, only ONE drug that targets a PPI has been approved. Continue reading
CARMA – Chimeric Antigen Receptor Macrophages
CARMA Therapeutics, a company that develops chimeric antigen receptor technology, not for T-cells (CAR-T cells), rather, for macrophages, hence the name “CARMA,” closed on an initial round of funding to advance its technologies. Continue reading
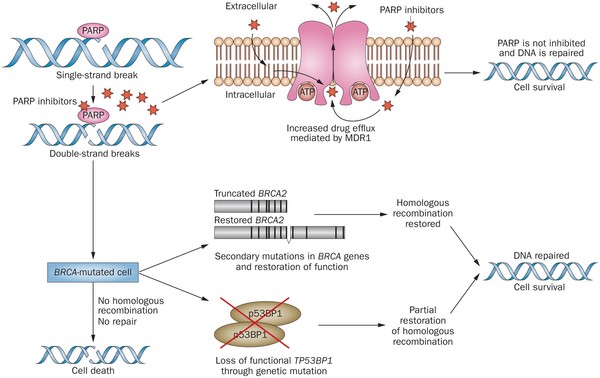
Olaparib – PARP inhibitor for triple negative breast cancer
Olaparib (Lynparza) is a PARP (poly-ADP ribose polymerase) inhibitor that was approved by the FDA in 2014 for the treatment of patients with advanced ovarian cancer who have mutated BRCA1,2 gene. Recently, the drug showed a 70% reduction in risk of progression in patients with less-advanced disease in the maintenance therapy setting:
The Phase III SOLO-2 trial demonstrated a significant improvement in progression-free survival (PFS) in germline BRCA-mutated (gBRCA), platinum-sensitive, relapsed ovarian cancer patients treated with Lynparza (olaparib) tablets (300mg twice daily) compared with placebo in the maintenance setting. The trial met its primary endpoint of investigator assessed PFS (HR 0.30; 95% CI 0.22 to 0.41; P<0.0001; median 19.1 months vs 5.5 months).
PARP inhibitors act in a counter-intuitive manner – by blocking PARP in the context of mutated BRCA1, the cell becomes overwhelmed with double strand breaks, leading to crisis and cell death. BRCA1 mutations, alone, predispose the cell to the accumulation of mutations in protooncogenes and tumor suppressor genes – a few double strand breaks are tumorigenic, whereas a massive number of double strand breaks, as occurs in the context of PARP inhibition, leads to apoptosis.
The use of PARP inhibitors for breast cancer makes great sense, However, in a Phase 3 trial of velparib, an experimental PARP inhibitor, failed to achieve better rates of complete pathogenic response in patients with triple negative breast cancer (TNBC – lack of HER-2, estrogen, and progesterone receptor up-regulation) versus chemotherapy, alone.
At the ASCO conference last week, AstraZeneca presented data on the use of olaparib in 302 patients with BRCA1,2 mutated breast cancer from its OlympiAD trial that compares olaparib against physician’s choice of chemotherapy (capecitabine 2500 mg/m2 d1-14 q 21, or vinorelbine 30 mg/m2 d1,8 q 21, or eribulin 1.4 mg/m2 d1,8 q 21):
OlympiAD Inclusion Criteria:
- Germline mutation in BRCA1 or BRCA2 that is predicted to be deleterious or suspected deleterious.
- Histologically or cytologically confirmed breast cancer with evidence of metastatic disease.
- Prior therapy with an anthracycline and a taxane in either an adjuvant or metastatic setting.
- Prior platinum allowed as long as no breast cancer progression occurred on treatment or if given in adjuvant/neoadjuvant setting at least 12 months from last dose to study entry elapsed.
- ER/PR breast cancer positive patients must have received and progressed on at least one endocrine therapy (adjuvant or metastatic), or have disease that the treating physician believes to be inappropriate for endocrine therapy.
- ECOG performance status 0-1.
- Adequate bone marrow, kidney and liver function.
OlympiAD Exclusion Criteria:
- Prior treatment with PARP inhibitor.
- Patients with HER2 positive disease.
- More than 2 prior lines of chemotherapy for metastatic breast cancer.
- Untreated and/or uncontrolled brain metastases.
Results were quite impressive – this was the first study that demonstrated PARP inhibition is effective in breast cancer:
- About 60% of patients saw their tumors shrink, a hair more than double the 29% objective response rate seen in those patients on chemotherapy.
- Lynparza showed efficacy in patients with TNBC, which is more difficult to treat. AbbVie, which is developing its own PARP inhibitor called veliparib, recentlyannounced a study specifically geared to look at veliparib’s activity in triple negative breast cancer failed to show a benefit when added to chemo.
- Additionally, treatment with Lynparza improved the time to second progression or death compared to chemo,suggesting patients who relapsed after Lynparza experienced a less aggressive return of their cancers.
Astrazeneca is studying olaparib with many combinations, including a study in TNBC with PD-L1 inhibitor durvalumab and CTLA-4 inhibitor tremelimumab.
Metformin improves cancer outcomes
Metformin (Glucophage) is an antihyperglycemic agent that lowers hepatic glucose production, improves peripheral glucose uptake and utilization, and does not cause increased insulin secretion. In fact, “with metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.” These properties have spawned a great deal of interest in metformin as a treatment for several types of cancers. Continue reading