Augmenting the responses to checkpoint inhibitors, which remove the “breaks” from the immune response, is a very popular area of research. The general concept is to turn immunologically cold tumors hot. For example, triple negative breast cancer (TNBC) is considered an immunologically cold tumor – anti-PD(L)1 therapy has shown responses of just 5-10%.
The TONIC trial is a phase 2 study in patients with TNBC who have received three or fewer prior lines of chemotherapy – Nivolumab After Induction Treatment in Triple-negative Breast Cancer (TNBC) Patients (TONIC). Patients are randomized to one of five induction regimens prior to receiving nivolumab (Opdivo):
- Radiation therapy – has been shown to induce immunogenic cell death (as evidence by abscopal responses to non-radiated sites), overcome T-cell exclusion, and promote antigen presentation;
- Low dose doxorubicin – increases production of interferon and reduces immune suppression mediated by myeloid-derived suppressor cells (MDSC);
- Low dose cyclophosphamide – depletes regulatory T-cells in breast cancer;
- Cisplatin – stimulates class I HLA molecule expression, thereby the vulnerability of tumor cells for T-cell killing;
- No induction – control arm (nivolumab, alone)
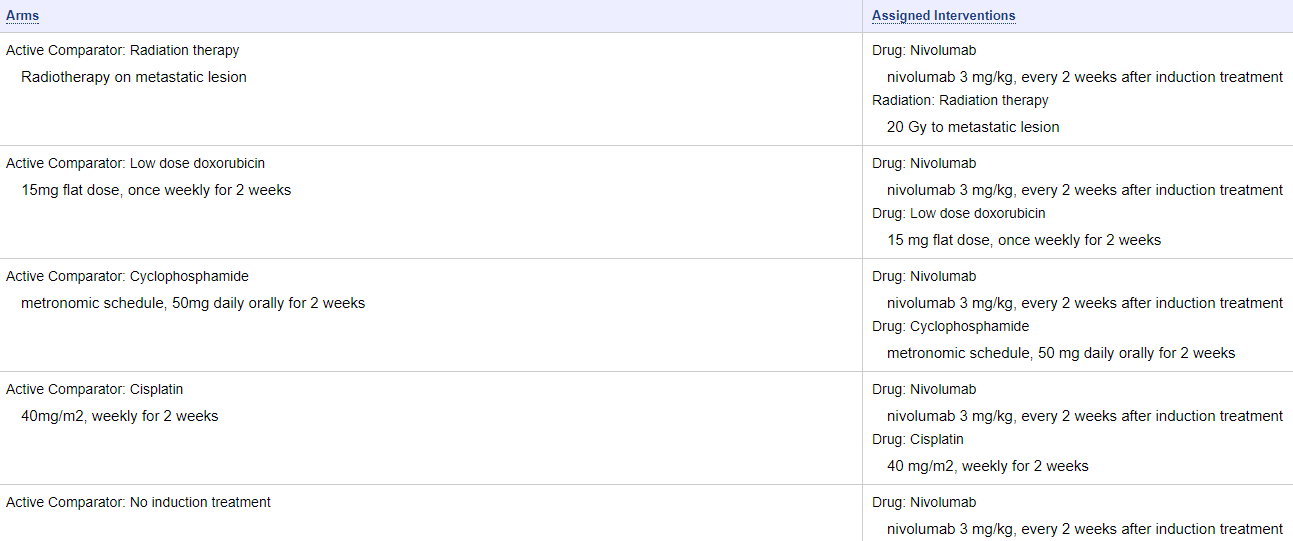
Figure 1. Study arms of TONIC trial. https://clinicaltrials.gov/ct2/show/NCT02499367
After the 2-week induction period, all patients received nivolumab at 3 mg/kg until progression. Fifty patients were evaluated for efficacy, with a median follow-up of 10.8 months:
Prior chemotherapy consisted of anthracyclines in 88% of patients, taxanes in 86%, platinum agents in 60%, and capecitabine in 54%. More than one fourth of patients (28%) received two or three prior lines of chemotherapy for metastatic disease. Forty-six percent of patients had PD-L1 expression ≥1% on tumor or immune cells.
The best objective response rate (ORR) was 24%, with one complete response (2%), 11 partial responses (22%), and one case of stable disease (4%), for a clinical benefit rate of 26%. The ORR by RECIST 1.1 was 22%. The median time to response was 2.1 months, and the median duration of response was 9.0 months. Median progression-free survival was 3.4 months (95% CI 2.5-3.7 months). Among patients with a complete or partial response, the 1-year overall survival rate was 83%, compared with 13% in the one patient who had stable disease.
When examined by induction arm, the ORRs were 10% with radiotherapy induction, 45% with doxorubicin, 11% with cyclophosphamide, 33% with cisplatin, and 11% with no induction. Most responses were observed after the start of nivolumab; only two responses occurred after the 2 weeks of immune induction.
The presence of TILs (tumor-infiltrating lymphocytes) was critical – the median level of TIL’s in the 11 responders was 23%, compared to just 5% in non-responders. The baseline CD8+ level was higher in responders than in non-responders (51 versus 26/mm2).
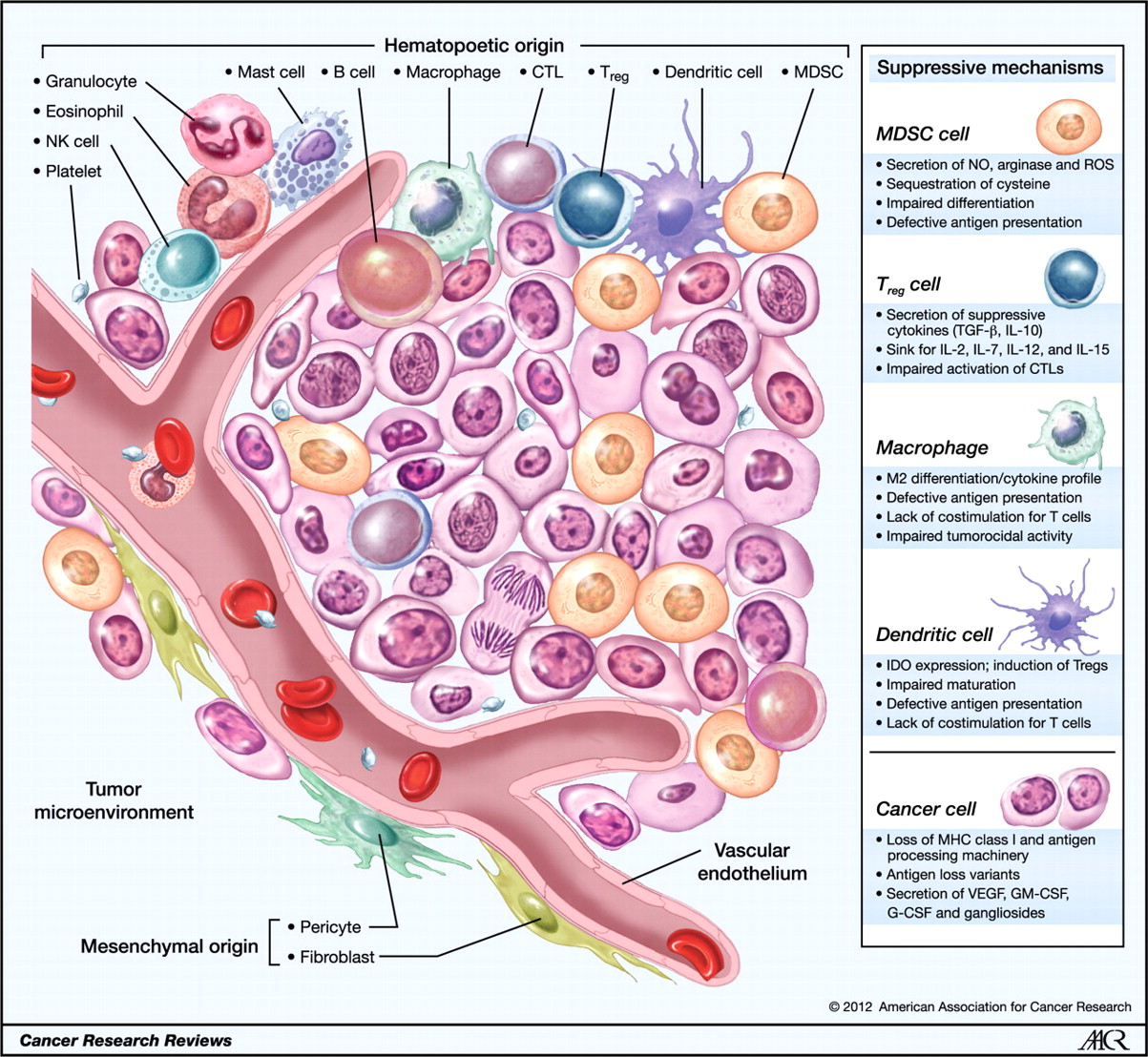
Figure 2. Cellular infiltrates within the tumor microenvironment. Established cancers consist of a wide array of immune cells that contribute to the tumor stroma of a growing malignancy. Tumors possess infiltrating cells of both innate and acquired immunity, such as MDSCs, macrophages, DCs, mast cells, eosinophils, neutrophils, NK cells, and lymphocytes. These cells coordinately form a complex regulatory network that fosters tumor growth by creating an environment that enables cancers to evade immune surveillance and destruction. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; NO, nitric oxide; ROS, reactive oxygen species. http://cancerres.aacrjournals.org/content/72/13/3125
Conclusion
Modulating the tumor microenvironment with the use of low dose doxorubicin or cisplatin (even in patients who have progressed or relapsed after receiving prior treatment with these agents) appears to be a viable strategy to condition patients for improved response to anti-PD(L)1 therapy. However, without sufficient TIL’s in the tumor microenvironment, immune conditioning via enhanced antigen presentation and depletion of Treg’s and MDSC’s is unlikely to provide benefit treatment with checkpoint inhibitors. Strategies to increase the presence of TIL’s in the tumor microenvironment should be employed in concert with immune priming and checkpoint therapy.
