Platelets are the second most abundant cellular component of blood. The platelet membrane contains an abundance of receptors to facilitate interactions with subendothelial matrix , other blood cells, and other platelets. The central role of platelets is in hemostasis, however, they also contain copious amounts of cytokines that induce inflammation.
Platelets possess toll-like receptors (TLRs), thereby having a significant role in immunity:
The platelet’s expression of Toll-like receptors (TLRs) indicates a capacity to directly engage microbial pathogens similar to leukocytes. Only a fraction of the platelet population is needed to maintain adequate hemostasis, suggesting that the excessive numbers allow platelets to act as major vascular surveyors of blood for recognizing foreign particles.
Platelets and cancer
Given the central role of inflammation as a cancer promoter, platelets are important contributors to tumorigenesis:
In 2000, Hanahan and Weinberg defined 6 hallmarks of cancer: (1) self-sufficiency in growth signals, (2) insensitivity to growth-inhibitory signals, (3) resisting cell death, (4) limitless replicative potential, (5) sustained angiogenesis, and (6) metastasis.In 2011, the list was updated with addition of other essential characteristics, such as cellular and microenvironment alterations necessary for malignant transformation, dysregulation of cell energetics, avoidance of immune destruction, genomic instability, and tumor-promoting inflammation. Indeed, many of these hallmarks resemble the inflamed state, placing the platelet within an interface that links thrombosis, inflammation, and cancer.
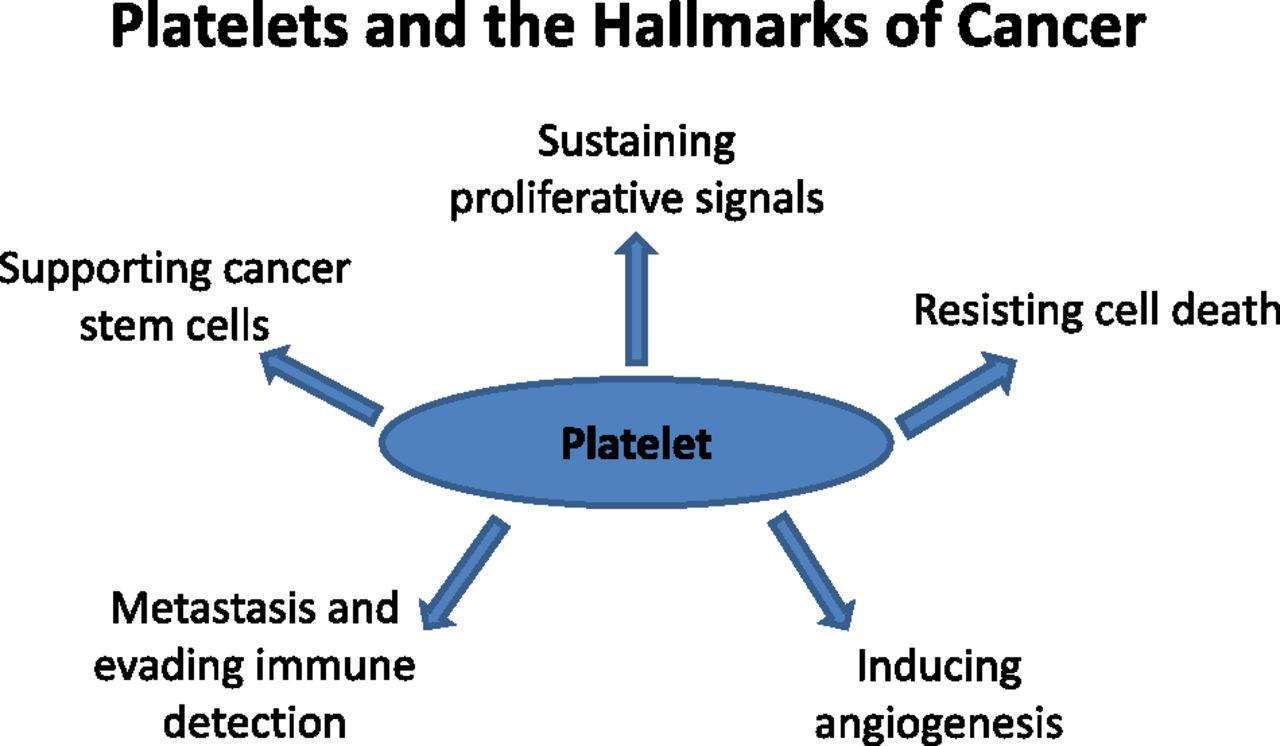
Figure 1. Platelets and the hallmarks of cancer. The original hallmarks of cancer included 6 biologic capabilities acquired during the complex development of tumors. Highlighted and discussed in the text are circulating platelet properties that contribute to some of the hallmarks. Thus, the platelet can be viewed as a normal cell contributing to the hallmark traits and influencing the TME. Antiplatelet therapies in the realm of cancer development and progression represent a future direction likely to impact patient prognosis and outcome. http://www.bloodjournal.org/content/126/5/582?sso-checked=true
Platelets interact with circulating tumor cells, protecting them from shear forces and immunological attack, and providing chemokine signals to maintain their viability during the metastatic process.
Exploiting platelets for targeted immunotherapy
After surgery for removal of primary cancers, the wound healing process is initiated. Local recurrence following surgery is commonplace, and adjuvant chemotherapy is often administered to kill residual cells in the tumor bed that were not removed surgically. However, due to anatomic alteration caused by the surgery, disrupted blood supply and hydrostatic pressure gradients can impede the delivery of drugs to the surgical area from the systemic circulation. However, platelets are recruited to surgical wound. Could platelets be used as a delivery vehicle for anti-cancer therapies?
Researchers conjugated anti-PD-L1 monoclonal antibodies to the surface of platelets and administered them to mice following surgical removal of melanoma and triple negative breast cancer:
Conjugating anti-PDL1 (engineered monoclonal antibodies against programmed-death ligand 1) to the surface of platelets can reduce post-surgical tumour recurrence and metastasis. Using mice bearing partially removed primary melanomas (B16-F10) or triple-negative breast carcinomas (4T1), we found that anti-PDL1 was effectively released on platelet activation by platelet-derived microparticles, and that the administration of platelet-bound anti-PDL1 significantly prolonged overall mouse survival after surgery by reducing the risk of cancer regrowth and metastatic spread. Our findings suggest that engineered platelets can facilitate the delivery of the immunotherapeutic anti-PDL1 to the surgical bed and target CTCs in the bloodstream, thereby potentially improving the objective response rate.
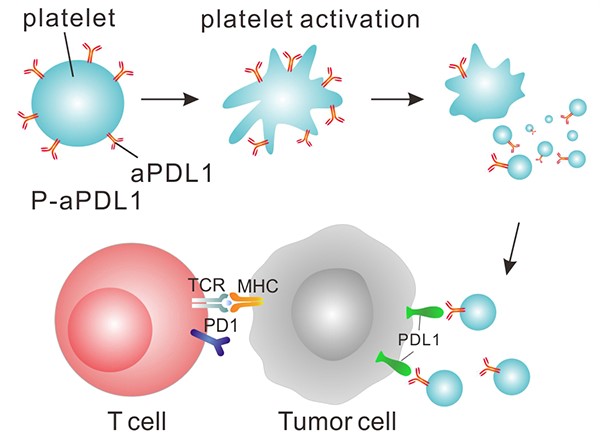
Figure 2. PD-L1 inhibitor-bound platelets (P-aPDL1) are targeted to the surgery wound, where they release the inhibitor and recruit immune system cells (T cells). Together, the inhibitor and T cells kill remaining tumor cells. https://www.cancer.gov/news-events/cancer-currents-blog/2017/platelet-immunotherapy
The engineered platelets release PD-L1 antibody, which mutes the immune-dampening stimulus(PD-L1) expressed on cancer cells, as well as cytokines that induce inflammation by recruiting other immune cells. The combination of activities provided for a very effective post-surgical adjunctive treatment. Moreover, the effects should be limited to areas where platelet activation is induced – the wound site, circulating tumor cells, and metastatic deposits:
The research team chemically linked a PD-L1 inhibitor to platelets they isolated from mice. They found that activation of the engineered platelets in the laboratory caused them to release the inhibitor along with the expected immune system-stimulating chemicals.
Next, they surgically removed approximately 99% of tumors in mice with melanoma, then injected the mice with inhibitor alone or the engineered platelets. Using fluorescent tags, the researchers found that, 2 hours after injection, the engineered platelets accumulated at the surgery wound site, whereas the inhibitor alone did not.
And, compared with mice treated with surgery only, mice treated with surgery and the engineered platelets had higher levels of immune system-stimulating chemicals and cancer-fighting immune cells at the wound site. However, these chemicals were not elevated in the bloodstream of mice treated with engineered platelets, indicating that the immune system activation was localized to the wound site.
Most importantly, compared to mice treated with normal platelets or the inhibitor alone, mice treated with the engineered platelets had the least amount of tumor regrowth after surgery, and they lived substantially longer.
To test whether the engineered platelets could prevent the formation of metastatic tumors, the researchers injected melanoma cells into the bloodstream of mice from whom tumors had been removed surgically. At the same time, they injected the mice with normal platelets, PD-L1 inhibitor alone, or engineered platelets.
After tracking the mice for about seven weeks, they found that only mice treated with the engineered platelets had reduced numbers of metastatic lung tumors as well as reduced growth of the original tumor. In addition, whereas none of the mice treated with normal platelets or inhibitor alone survived past 40 days, about half of the mice treated with the engineered platelets were still alive at that time.
The researchers observed similar results when they treated mice bearing an aggressive type of breast cancer with the engineered platelets.
