Juno Therapeutics is developing Chimeric Antigen Receptor (CAR) T-cells directed against B-cell antigen CD19 for the treatment of patients with B-cell lymphomas. The company has elected to halt the development of JCAR015 for Acute Lymphoblastic Leukemia (ALL) and proceed with JCAR017 for relapsed/refractory diffuse large B-cell lymphoma (DLBCL), the most common form of Non-Hodgkin Lymphoma (NHL), due to the development of cerebral edema and subsequent death of several patients with ALL enrolled in its phase 2 ROCKET trial, which has been suspended.
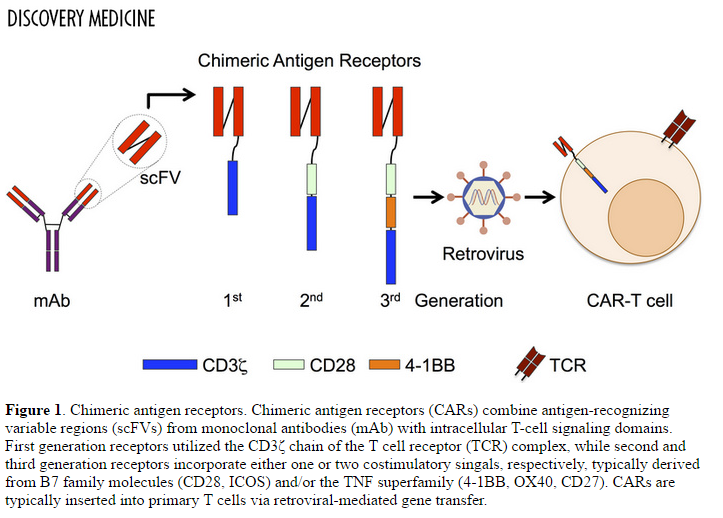
Chimeric Antigen Receptor T-cells
CAR T-cells have evolved from first generation constructs that contained an extracellular binding domain and intracellular signaling domain, to third generation structures that also include co-stimulatory molecules.
CD19
JCAR015 and JCAR017 both target the CD19 antigen, a 95 kilodalton glycoprotein belonging to the immunoglobulin class. No significant homology exists between CD19 and other known proteins, making it an ideal biomarker for B-cells.
CD19 acts as a critical co-receptor for BCR signal transduction. As BCR (B-cell receptor) signaling requires protein tyrosine kinase (PTK) activation, CD19 recruits and amplifies the activation of Src-family protein tyrosine kinases such as Lyn and Fyn. Upon BCR activation, CD19 also enhances BCR-induced signaling crucial for B cell expansion, through recruitment and activation of PI3K and downstream Akt kinases.
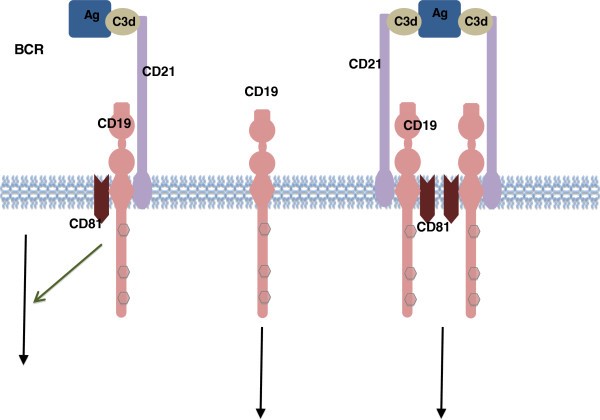
Figure 2. CD19 associated signaling complex. Antigen-C3d complexes can engage the CD19/21 complex in both a BCR-independent or BCR-dependent fashion. The CD19 complex includes complement receptor CD21, which binds C3d-modified antigen. Rituxan (rituximab), a monoclonal antibody for the treatment of Non-Hodgkin’s Lymphoma (DLBCL) and Chronic Lymphocytic Leukemia, binds to CD19.
JCAR015 versus JCAR017
These Chimeric Antigen Receptors are comprised of four domains: (1) extracellular targeting – single chain variable fragment; (2) transmembrane; (3) cytoplasmic costimulatory; and (4) cytoplasmic signaling domain.
Upon recognition and binding of the scFv of the CAR T cell to the cancer cell, there is a conformational change that leads to an activation signal to the cell through CD3-zeta, an intracellular signaling protein. Our current CAR constructs also include either a CD28 or 4-1BB costimulatory signaling domain to mimic a “second signal” that amplifies the activation of the CAR T cells, leading to a more robust signal to the T cell to multiply and kill the cancer cell.
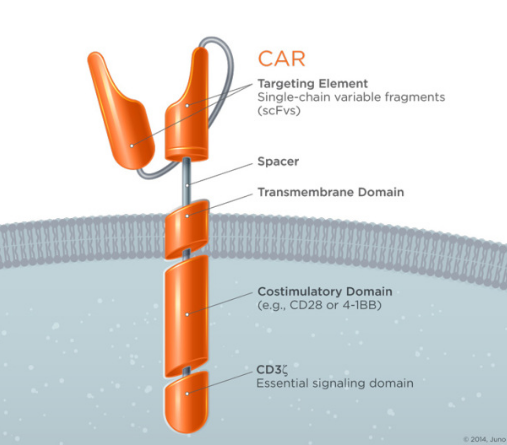
Figure 3. Template of JCAR015 and JCAR017. https://www.junotherapeutics.com/our-science/car-technology/
There are important differences between JCAR015 and JCAR017, which likely account for the improved safety profile of the latter:
- The cells used to create JCAR015 are composed of CD3+ enriched peripheral blood mononuclear cells, whereas JCAR017 is built using a fixed ratio of CD4+ and CD8+ T-lymphocytes.
- Each agent is engineered using different viral vectors, namely gamma retroviral for JCAR015 and lentiviral for JCAR017.
- The binding domain for JCAR015 is SJ25C1 and the costimulatory domain is CD28, whereas JCAR017 has a binding domain of FMC63 and a costimulatory domain of 4-1BB.
- JCAR017 contains an ablative technology to provide better control of proliferation and survival of the engineered T cells, which is not included in JCAR015. This ablative technology, a truncated form of the human epidermal growth factor receptor (EGFRt), allows for rapid killing of the CAR T-cells using cetuximab (Erbitux), if needed. The addition of EGFRt may also have immunostimulating properties.
By including only CD4+ (T-helper) and CD-8+ (Cytotoxic T-cells), the immune attack is more focused – CD3+ positive cells also include macrophages. Co-stimulatory molecule CD28 stimulates the B7 signaling pathway, which results in stabilization of cytokine mRNA, whereas, 4-1BB triggers the tumor necrosis factor receptor (TNF-R)-associated factor (TRAF) pathway, which results activation of NFkB . CAR T-cells with CD28 expand rapidly, whereas, those with 4-1BB expand more slowly – this is thought to contribute to improved tolerability, including less cerebral edema.

Figure 4. Biochemical and transcriptional effects of the CD28 signaling pathways. Summary of biochemical signaling events downstream of the proximal and distal CD28 motifs that can modulate the T-cell transcription pattern after co-stimulation. Among the different mechanisms, we can distinguish a direct effect on gene expression by regulation of transcription factors or an indirect effect through increases in mRNA stability. https://www.researchgate.net/figure/51047463_fig3_Figure-1-Biochemical-and-transcriptional-effects-of-the-CD28-signaling-pathways-Summary
CAR T-cells incorporating 4-1BB are more persistent and less likely to undergo exhaustion than those incorporating CD28. This probably contributes to improved effectiveness seen with CAR017. Inclusion of EGFR in the construct adds a great measure of safety – the ability to neutralize the CAR T-cells is critical should adverse events emerge.

Figure 5. 4-1BB signaling. http://rgcb.res.in/orcadb/biocarta_analysis.php?gene_id=7018&gene_symbol=TF
Clinical results with JCAR017
JCAR017 has shown impressive results in the clinic to data. In a phase 1 study in patients with NHL, a 60% complete response (CR) rate and an 80% overall response rate were observed. None of the patients experienced severe cytokine release syndrome (CRS) and fourteen percent experienced neurotoxicity, which resolved with treatment.
In a phase 1 study of young patients with relapsed/refractory CD-19 positive ALL, the minimal residual disease (MRD)-negative CR rate was 93%. In those pre-treated with fludarabine and cyclophosphamide, the MRD-negative CR rate was 100%. Twenty-three percent experienced severe CRS, and grade 3 neurotoxicity was seen in 23% of patients.
In adults with ALL, the CR rate was 77% and the MRD-negative rate was 90% (in those who could be evaluated for MRD). Twenty-seven percent of patients experienced severe CRS, and twenty-nine percent had grade 3 or 4 neurotoxicity.