Procaspase-3 is an executioner protein catalyzes the hydrolysis of more than 100 protein targets. These cleavage events ultimately lead to cell suicide, or apoptosis. Caspase-3 is triggered by the intrinsic and extrinsic apoptosis cascades.
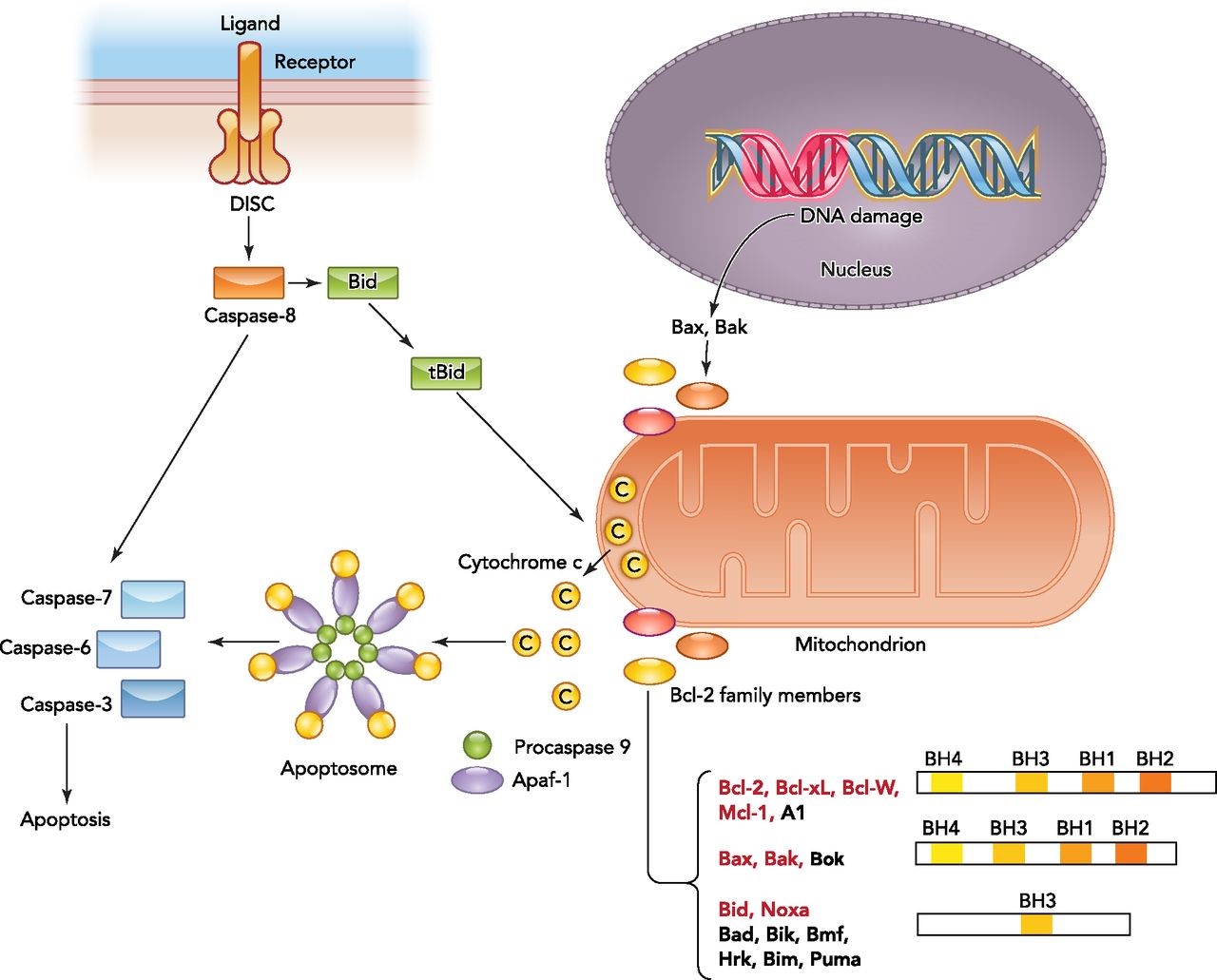
Overview of the intrinsic and extrinsic apoptotic signaling pathways
The binding of a death ligand to the death receptor initiates the extrinsic pathway and results in activation of the death-inducing signaling complex (DISC). Caspase-8 then either directly activates the downstream caspase (type I cells) or needs an amplification step (type II cells) via cleavage of Bid. Intrinsic apoptotic signaling occurs in response to physiological signals or cellular stresses such as DNA damage. Upon activation, the balance between antiapoptotic and proapoptotic Bcl-2 members on the mitochondrial membrane shifts and results in outer mitochondrial membrane permeabilization and cytochrome c release. Bcl-2 family members targeted by HIF are highlighted in red. Cytosolic cytochrome c binds to the apoptotic caspase activating factor (Apaf1) and recruits procaspase-9 to form the apoptosome. Activated caspase-9 within the apoptosome can then promote activation of downstream caspases. http://physiologyonline.physiology.org/content/29/3/168
Most tumors have disruptions in the protein signal cascades that produce active caspase-3. These disruptions thwart cell suicide and allow the cancer to grow unchecked. Cancer cells have key aberrations in the apoptotic pathways enabling them to resist both intrinsic (through restriction of p53) and extrinsic (through decrease procaspase-8 expression) apoptotic triggers.
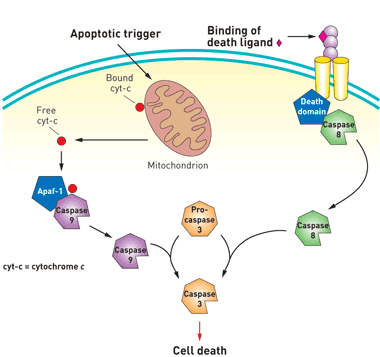
Apoptosis can be initiated through one of two cell-signaling pathways. Both pathways converge on caspase-3, a protein that acts as the cell’s executioner. PAC-1 (Procaspase Activating Factor-1, see below) bypasses both pathways and initiates apoptosis by inducing procaspase-3 to cleave itself to yield active caspase-3. https://pubs.acs.org/cen/news/84/i38/8438molecule.html
![Western Blot: Caspase-3 (Pro and Active) Antibody [NB100-56112] - analysis of Caspase-3. Lysates from Jurkat cells (leukemic T-cell line - lane 1), normal mammary tissue (lane 2) and surgical specimens from three invasive ductal carcinomas (lanes 3-5) were normalized for total protein content (50 ug/lane) and western blotted with anti-Caspase-3. The ~32 kDa pro-Caspase-3 protein was detected in all samples. Active/cleaved Caspase-3 was identified in Jurkat and two ductal carcinomas (14-21 kDa large subunit). http://www.novusbio.com/Caspase-3-Antibody_NB100-56112.html](http://blogs.shu.edu/cancer/files/2016/02/Caspase-3-Pro-and-Active-Antibody-Western-Blot-NB100-56112-img0005.jpg)
Western Blot: Caspase-3 (Pro and Active) Antibody [NB100-56112] – analysis of Caspase-3. Lysates from Jurkat cells (leukemic T-cell line – lane 1), normal mammary tissue (lane 2) and surgical specimens from three invasive ductal carcinomas (lanes 3-5) were normalized for total protein content (50 ug/lane) and western blotted with anti-Caspase-3. The ~32 kDa pro-Caspase-3 protein was detected in all samples. Active/cleaved Caspase-3 was identified in Jurkat and two ductal carcinomas (14-21 kDa large subunit). http://www.novusbio.com/Caspase-3-Antibody_NB100-56112.html
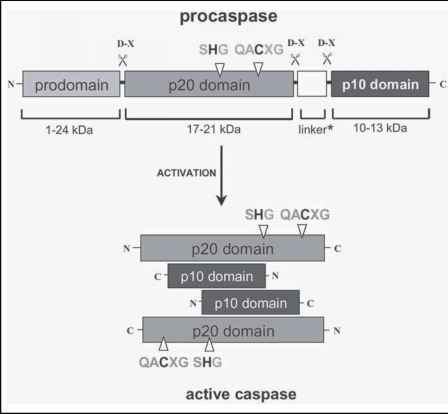
A caspase zymogen exists of a prodomain of variable length (5 to 219 amino acids) followed by a p20 and a p10 domain. (*) A peptide linker separates the latter two domains in caspase-1, -2, -4, -5, -6, -8 and -9. The diagram depicts the position of the catalytic cysteine and histidine residues in the p20 domain and the aspartate-X bonds that are cleaved during the proteolytic maturation of the enzyme. Mature caspase is a heterotetramer, with each heterodimer consisting of a p20 and a p10 subunit. http://www.clicktocurecancer.info/caspase-activation/threedimensional-structure-of-mature-caspases.html
PAC-1 likely acts by interfering with one of caspase-3’s safety features. The protein is normally in an inactive form called procaspase-3 until a domain of the protein is chopped off, thereby creating active caspase-3. A further three-amino-acid safety catch prevents procaspase-3 from activating itself by cleaving itself. PAC-1 is believed to work by interfering with this safety catch, thereby allowing procaspase-3 to activate itself and initiate cell death.
Unlike inhibitors that work stoichiometrically, PAC-1 is a catalytic activator, Hergenrother (University of Illinois, Urbana-Champaignsaid). “This is a benefit. One molecule of PAC-1 will activate procaspase-3 to produce caspase-3. Then PAC-1 is free to activate another one.”
“This is a novel strategy for a cancer therapy,” commented Michael F. Olson of the Beatson Institute for Cancer Research in Glasgow, Scotland. “Going straight to one of the key proteins that actually kills the cell and directly activating it could induce the death of cancer cells that are insensitive to many forms of standard chemotherapy.”
Initial research with patient tissue samples and mouse models shows that PAC-1’s ability to destroy cancer cells is related to the levels of procaspase-3 in a cell, Hergenrother said. “Since tumor procaspase-3 levels can be measured, one might be able to predict a priori what types of patients would respond to a procaspase-3 activator. This is a step toward personalized cancer therapy.”
A clinical trial was initiated last year – the Phase 1 dose escalation trial, which is still recruiting patients, is being evaluated in a number of cancers: Breast, gastrointestinal, genitourinary, gynecologic, head and neck, thoracic, as well as lymphomas, melanomas and solid tumors.