Elotuzumab (Empliciti) is now the second monoclonal antibody (Mab) approved by the FDA for multiple myeloma (MM). Darzalex (daratumumab), the first Mab for MM, was approved by the FDA just 3 weeks ago.
The FDA approved Empliciti to be used in combination with two other FDA-approved treatments, dexamethasone and Celgene Corp.’s Revlimid. In a 646-participant clinical trial, those treated with the three-drug cocktail took longer to relapse and had a higher chance of tumor shrinkage than those treated with the two-drug therapy.
The safety and efficacy of Empliciti were tested in a randomized, open-label clinical study of 646 participants whose multiple myeloma came back after, or did not respond to previous treatment. Those taking Empliciti plus Revlimid and dexamethasone experienced a delay in the amount of time before their disease worsened (19.4 months) compared to participants taking only Revlimid and dexamethasone (14.9 months). Additionally, 78.5 percent of those taking Empliciti with Revlimid and dexamethasone saw a complete or partial shrinkage of their tumors compared to 65.5 percent in those only taking Revlimid and dexamethasone.
Multiple myeloma is a type of blood cancer that occurs white blood cells found in the bone marrow. The National Cancer Institute estimates there will be 26,850 new multiple myeloma cases and 11,240 related deaths in the U.S. this year. Empliciti activates the body’s own immune system to attack and destroy multiple myeloma cells.
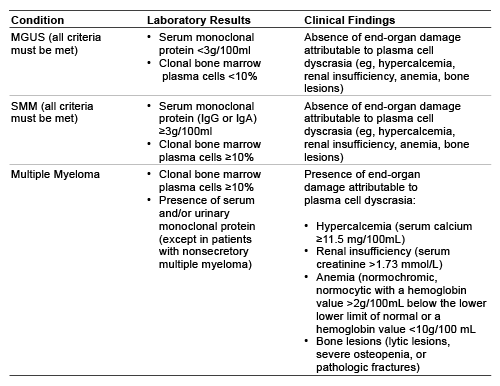
Multiple myeloma may be preceded by either monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma (SMM). MGUS is a premalignant condition that is associated with a 1% per year progression to multiple myeloma. Smoldering multiple myeloma (SMM) resembles MGUS in the absence of end-organ involvement, but is much more likely to progress to myeloma.
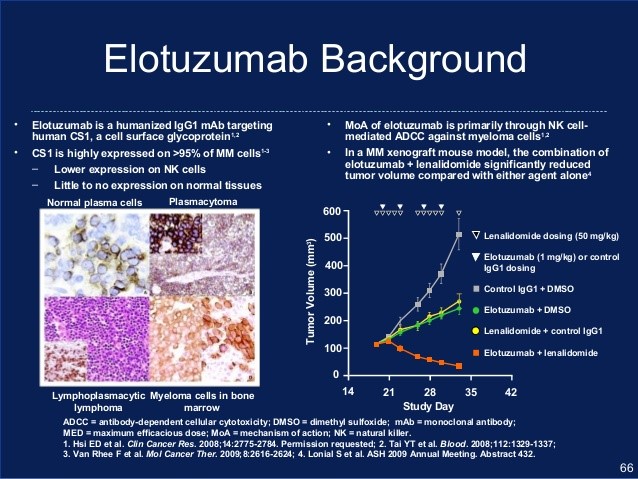
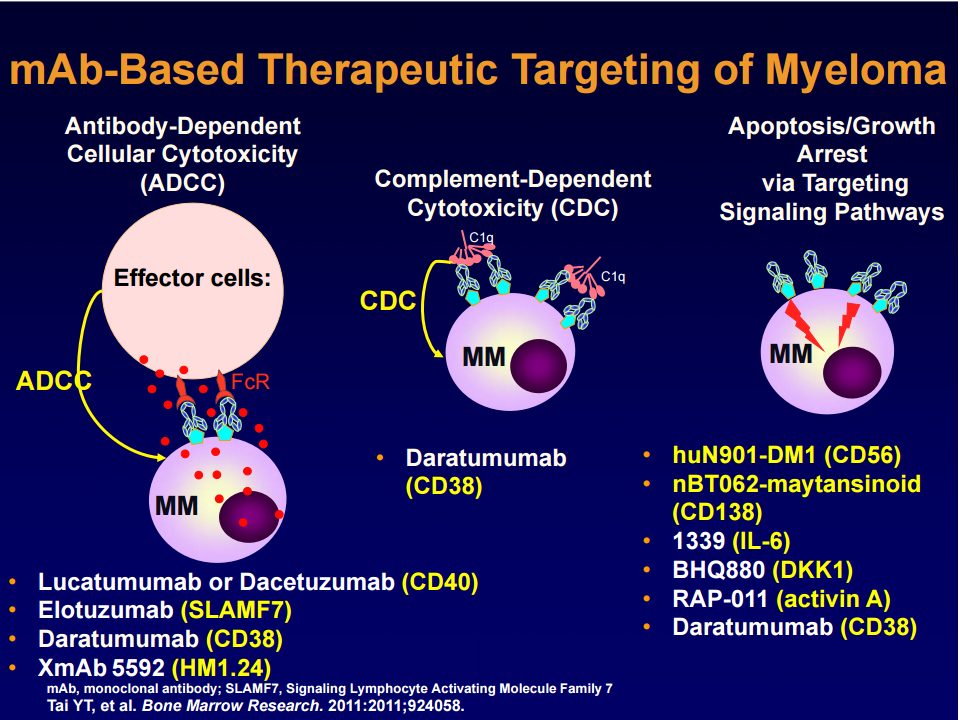
Elotuzumab is a humanized monoclonal antibody that binds to CS1 (SLAMF7), which is expressed on MM cells, as well as NK cells. It opsonizes MM for destruction by NK cells, and also activates NK cells.
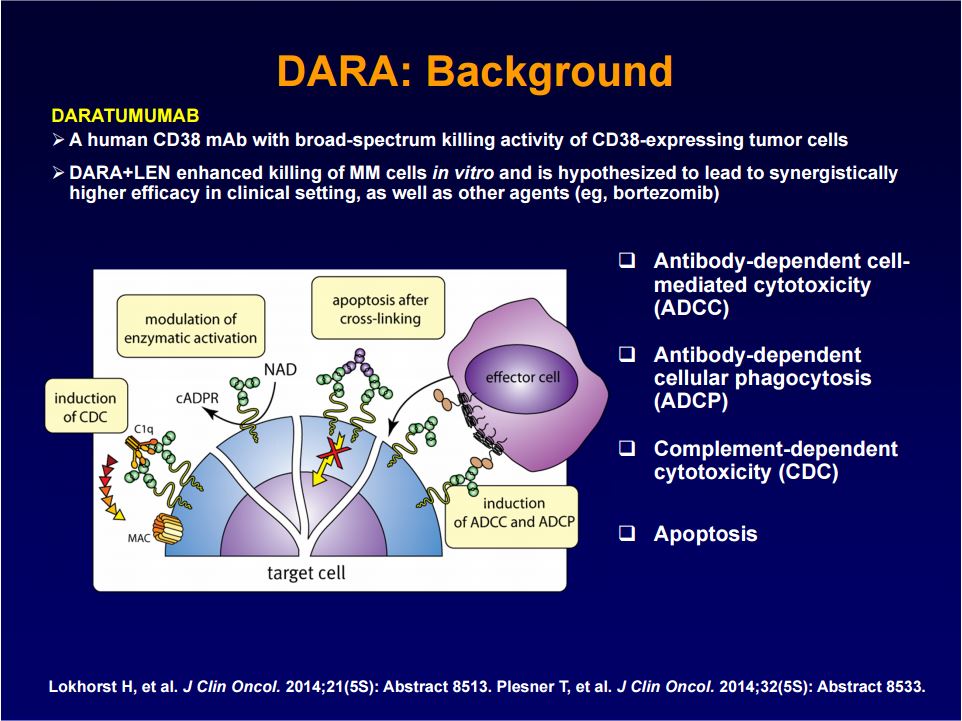
Darzalex binds to CD-38, a multifunctional ectoenzyme that catalyzes the conversion of nicotinamide adenine dinucleotide (NAD+) into nicotinamide, adenosine diphosphate–ribose (ADPR), and cyclic ADPR. On terminally differentiated plasma cells, CD-38 is one of the few surface antigens present.
OncLive spoke with Sagar Lonial, MD (professor, executive vice chair, Department of Hematology and Medical Oncology, Emory University School of Medicine, chief medical officer, Winship Cancer Institute), who has performed clinical trials on both elotuzumab and daratumumab, to learn more about the future of monoclonal antibodies in multiple myeloma.
“Monoclonal antibodies offer a unique and different action from what we are used to using. This new mechanism of action is very important for those patients who don’t have other options. In the newly diagnosed population, it may offer us a higher percentage of patients who are cured.”