Liquid biopsy is the use of blood to diagnose cancer. Pathway Genomics launched its CancerIntercept test that screens for multiple cancers.
There are two versions of the CancerIntercept test. The Detect test is for circulating tumor DNA (ctDNA) strictly in high-risk but otherwise healthy patients, for example, the 50-year-old regular smoker. The other test, called the CancerIntercept Monitor, keeps tracks patients who have active or previously diagnosed cancer.
Note that contrary to the statements in the article cited above (“That means that from a single blood test it will be able to detect whether otherwise healthy person has cancer”), these are not healthy, asymptomatic screening populations.
The FDA has recently challenged the company – “We have […] examined published literature and have not found any published evidence that this test or any similar test has been clinically validated as a screening tool for early detection of cancer in high risk individuals,” the letter states. “We believe you are offering a high risk test that has not received adequate clinical validation and may harm the public health.” Disturbingly, none of the evaluations performed by the company would actually be able to prove that the blood test works. Pathway Genomics hasn’t completed a long-term study that shows that a positive test result leads to a cancer diagnosis.
The tests look for genetic markers (deletions, translocations, amplification, etc.) in oncogenes BRAF, EGFR, KRAS, GNAS, NRAS, PIK3CA, and tumor suppressor genes CTNNB1 (Cadherin-Associated Protein), FOXL2, and TP53.
It is important to emphasize that these tests are NOT for screening of healthy, asymptomatic patients, without either risk factors for developing cancer, or a personal or family history of cancer. Rather, they are intended to monitor patients with cancer who have undergone treatment or for patients at high risk for developing cancer on the basis of family history or the development of pre-malignant conditions.
Why?
Because they are not perfect, that is, they are not 100% specific because they are not based on pathognomonic signatures of disease versus healthy tissue. And when diagnostic tests that are not based on a parameter(s) that is exclusive to diseased tissue are not highly specific, they should limited to patients for whom the pre-test probability of disease is high because a positive test is likely to mean that disease is present in these circumstance. When the pre-test probability of disease is low, the likelihood that a positive test is a “false positive” is high (see below).
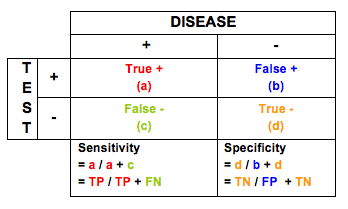
Sensitivity and specificity of diagnostic tests describe their performance in detecting disease and successfully ruling-out disease.
Sensitivity is the ability of a test to detect disease when disease is truly present. Mathematically, it is defined as True Positives (a) divided by True Positives (a) + False Negatives (c). Thus, the enemy of sensitivity is missing disease when it is present (false negative).
The implications of a test that is not very sensitive is missing disease. However, in circumstances where early detection is not possible, a test with relatively low sensitivity can be very medically useful, provided its specificity is high. This is the case because at the very least, more disease will be detected than is currently occurring. However, if the specificity is low, the cost in dollars and medical implications (surgeries, toxicity from treatment) do not warrant the gain in detection.
Specificity is the ability to successfully rule-out disease when no disease is present. Mathematically, it is defined as True Negatives (d) divided by True Negatives (d) + False Positives (b). Thus, the enemy of specificity is falsely declaring that disease is present when it really is not. Tests with low specificity lead to unnecessary medical testing, procedures, and treatments, all of which can have significant costs in terms of dollars and morbidity and mortality.
Related to specificity is Positive Predictive Value (PPV) – True Positives (a) divided by True Positives (a) + False Positives (b). This provides insight into the proportion of positive tests that truly indicate the presence of disease. The enemy of PPV is False Positives. However, PPV considers the benefits of true positives in balancing out the downside of false positives.
Consider these measures with respect to PSA screening for prostate cancer:
Sensitivity and specificity — The traditional cutoff for an abnormal PSA level in the major screening studies has been 4.0 ng/mL. The American Cancer Society systematically reviewed the literature assessing PSA performance. In a pooled analysis, the estimated sensitivity of a PSA cutoff of 4.0 ng/mLwas 21 percent for detecting any prostate cancer and 51 percent for detecting high-grade cancers (Gleason ≥8). Using a cutoff of 3.0 ng/mL increased these sensitivities to 32 and 68 percent, respectively. The estimated specificity was 91 percent for a PSA cutoff of 4.0 ng/mL and 85 percent for a 3.0 ng/mL cutoff. PSA has poorer discriminating ability in men with symptomatic benign prostatic hyperplasia.
Positive predictive value — The test performance statistic that has been best characterized by screening studies is the positive predictive value: the proportion of men with an elevated PSA who have prostate cancer.
Overall, the positive predictive value for a PSA level >4.0 ng/mL is approximately 30 percent, meaning that slightly less than one in three men with an elevated PSA will have prostate cancer detected on biopsy. For PSA levels between 4.0 to 10.0 ng/mL, the positive predictive value is about 25 percent; this increases to 42 to 64 percent for PSA levels >10 ng/mL.
However, nearly 75 percent of cancers detected within the “gray zone” of PSA values between 4.0 to 10.0ng/mL are organ confined and potentially curable. The proportion of organ-confined cancers drops to less than 50 percent for PSA values above 10.0 ng/mL. Thus, detecting the curable cancers in men with PSA levels less than 10.0 ng/mL presents a diagnostic challenge because the high false-positive rate leads to many unnecessary biopsies.
Any choice of PSA cutoff involves a tradeoff between sensitivity and specificity. While lowering the PSA cutoff would improve test sensitivity, a lower PSA cutoff would also reduce specificity, leading to far more false-positive tests and unnecessary biopsies. It has been projected that if the PSA threshold were to be lowered to 2.5 ng/mL, the number of men defined as abnormal would double, to up to six million in the US. Additionally, many of the cancers detected at these lower levels may never have become clinically evident, thereby leading to over-diagnosis and over-treatment.
Although screening for prostate cancer with PSA can reduce mortality from prostate cancer, the absolute risk reduction is very small. Given limitations in the design and reporting of the randomized trials, there remain important concerns about whether the benefits of screening outweigh the potential harms to quality of life, including the substantial risks for overdiagnosis and treatment complications. Men who are willing to accept a substantial risk of morbidity associated with treatment in return for a small reduction in mortality might reasonably choose to be screened. Men who are at increased risk of prostate cancer because of race or family history may be more likely to benefit from screening.
The same considerations are relevant with respect to the use of screening mammography in breast cancer detection for women at various levels of risk. Early detection of breast cancer with screening mammography means that treatment can be started earlier in the course of the disease, possibly before it has spread. Results from randomized clinical trials and other studies show that screening mammography can help reduce the number of deaths from breast cancer among women ages 40 to 74, especially for those over age 50. However, studies to date have not shown a benefit from regular screening mammography in women under age 40 or from baseline screening mammograms (mammograms used for comparison) taken before age 40.
The pre-test probability of disease modulates the performance of the test, as well as its interpretation. Screening mammography has a sensitivity of about 85 per cent in women over 50 and about 70 per cent in women aged between 40 and 49. Specificity is about 95 per cent – that is, about 5 per cent of women without cancer will require some further investigation. Ultrasound has a sensitivity of about 85 per cent and a specificity of 90 per cent in detecting blockage in the arteries to the brain. However, ultrasound has a sensitivity of only about 60 per cent and a specificity of 97 per cent for detecting clots in the veins in the legs after operations. Of course, one can also assess the accuracy of symptoms and the signs. For example, if you are admitted to hospital with possible appendicitis, the pain in the bottom right part of your abdomen has a sensitivity of 81 per cent and a specificity of 53 per cent.
So, how should the CancerIntercept tests be used if NOT for screening asymptomatic patients? Here are the ways that the test is being marketed. Clinical studies on these applications will elucidate the medical value of testing:
Tumor Profiling For Precision Medicine
The CancerIntercept™ Monitor test can provide physicians with valuable information about a patient’s tumor profile (somatic mutations present in the tumor), which can be utilized in the development of a personalized treatment plan.
Monitor Disease Progression & Tumor Evolution
While patients are undergoing cancer treatment, oncologists can use the CancerIntercept™ Monitor test to check the development of the patient’s tumor progression and/or tumor evolution (changes in the type of mutations within a tumor). It is important to evaluate tumor evolution throughout treatment as it can lend information about potential drug sensitivity and resistance.
Monitor Residual Disease and/or Recurrence
In instances where patients have undergone a resection of their tumor and/or have gone into disease remission, serial analysis of ctDNA burden utilizing the CancerIntercept™ Monitor test, can help check the development of disease re-occurrence or progression.
Clinical Trial Matching
Clinical Trial Matching is an additional feature of the CancerIntercept™ Monitor test, which gives your patient the option to receive personalized information about clinical trials that may be best suited for him or her, based on their tumor’s profile.
Appropriate Candidates for Testing
CancerIntercept™ Detect is specifically designed for individuals that are at high risk to develop cancer in their lifetime. There are a number of reasons that an individual may have an increased risk for cancer, including, but not limited to:
1 – Known predisposition to a hereditary cancer syndrome (i.e. the individual carries a BRCA1pathogenic variant)
2 – A family history of cancer (i.e. a mother diagnosed with colon cancer)
3 – Lifestyle choices (i.e. a history of smoking)
4 – Environmental exposures (i.e. previous exposure to radiation)