Immatics, a German biotechnology company has established a subsidiary in Houston, TX to collaborate with MD Anderson on overcoming some of the limitations to the adoptive T cell therapies now in the immuno-oncology pipeline. The collaboration covers the use of cytokine IL-21 to expand high-quality T cell batches, particularly those generated by CAR-T (chimeric antigen receptor T-cell) and related approaches.
What is IL-21?
IL-21 belongs to the family of gamma-chain receptor cytokines that includes IL-2, IL-7, and IL-15—cytokines that all deliver their intracellular signal through the shared gamma-chain receptor and influence T-cell activation and differentiation.
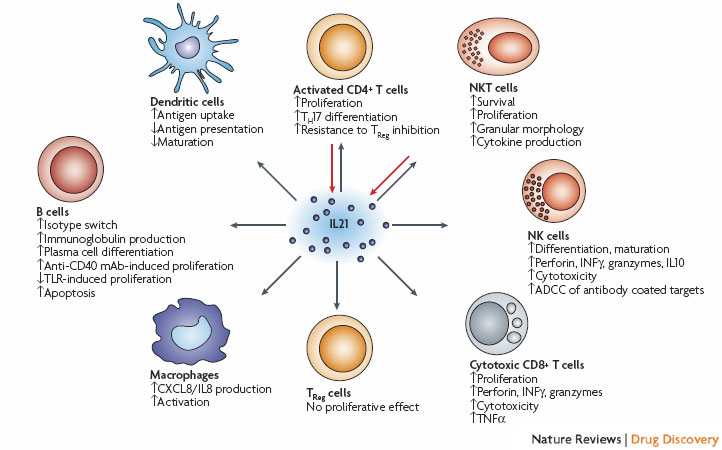
Interleukin 21 (IL21) is secreted by activated CD4+ T cells, T-follicular helper cells and natural killer T (NKT) cells19, 20, 21, and is able to modulate the activity of most lymphocyte subsets. The listed effects on CD4+ T cells19, 119, 120, 121 and CD8+ T cells19, 26, 33,45, 55 have been observed after IL21 stimulation together with T-cell receptor (TCR) stimulation or other activating cytokines (IL2, IL15), whereas the effects on NK cells19, 24, 39, 43, 44 also require other activating cytokines or activation through Fc receptors. It has been reported that IL21 does not have any direct effects on regulatory T (TReg) cells in mice35, but does suppress FOXP3 in human CD4+ T cells32. Other B-cell stimulatory agents (cytokines, immunoglobulins, Toll-like receptor (TLR) agonists, CD40 ligation) is also required for the listed effects on B cells19, 27, 119, 122, 123, 124, 125, 126, 127, dendritic cells128, 129 and macrophages130, 131. ADCC, antibody-dependent cellular cytotoxicity; IFN , interferon- ; mAb, monoclonal antibody; TH17, T-helper cell 17; TNF , tumour necrosis factor- . http://www.nature.com/nrd/journal/v7/n3/fig_tab/nrd2482_F1.html
What problem can IL-21 help address in cancer immunotherapy?
One major obstacle to adoptive therapy has been the feasibility of isolating tumor-reactive T cells. In addition, T cells may be suppressed by circulating regulatory cells (Tregs), that can lead to impaired proliferative response to antigenic stimulation.
In animal models, tumor antigen–specific CD8 cells failed to undergo normal functional maturation in the presence of Tregs and were rendered incapable of destroying specific tumor targets. Conversely, depletion of regulatory T cells controlled the growth of melanoma in most mice and promoted long-lasting CD8+ T-cell–dependent protective immunity, possibly through the recruitment of high-avidity antigen-specific cytotoxic T lymphocytes (CTLs). Recent evidence of elevated Tregs in the peripheral blood of patients with cancer and the finding that increased prevalence of tumor-associated Tregs in situ as a predictor for reduced survival suggest the importance of regulatory control of the endogenous antitumor immune response. The suppressive mechanisms at play in vivo may also limit the capacity to generate antigen-specific T cells in vitro.
Expression of the forkhead transcription factor, Foxp3, has been linked to the regulatory (Treg) phenotype. CD4+cells constitutively expressing CD25hi are also Foxp3+. Depletion of CD25+ cells in vitro can lead to enhanced generation of CD4+ T cells recognizing tumor-associated self-antigens, presumably by eliminating the inhibitory influence of CD25+ Tregs in the peripheral blood mononuclear cell (PBMC) responder population.
What is FOXP3?
The discovery of Foxp3 as the key transcription factor controlling T regulatory cell (Treg) development and function represents one of the most significant advances in Treg immunobiology. While the complete function and differentiation of Tregs now appears to require the presence of additional transcription factors, including AhR and STAT-5, it remains clear that Foxp3 is the “master-regulator” for the Treg lineage. Induction of the Foxp3 gene in normal naïve T cells converts them to Treg-like cells with in vivo and in vitro suppressive function, indicating that Foxp3 is likely to play a key role in controlling expression of critical suppression-mediating molecules.
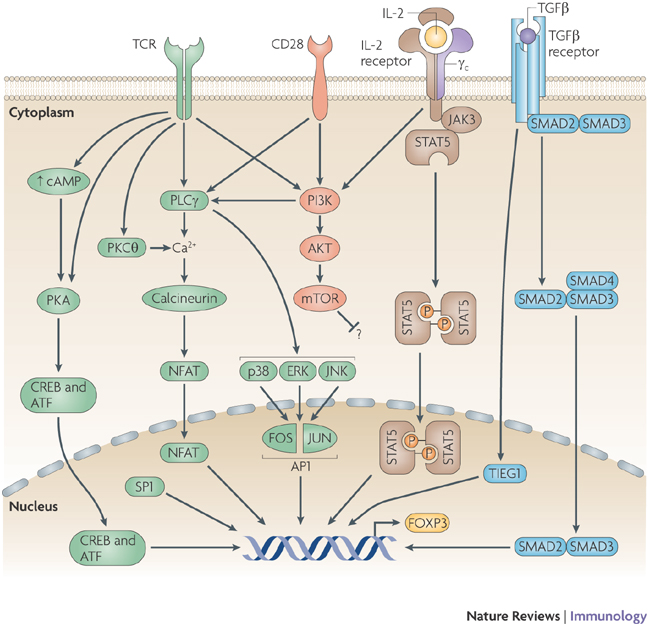
Signals that are triggered following ligand binding to the T-cell receptor (TCR), CD28, cytokine receptors that contain common cytokine-receptor -chain ( c; here represented by the interleukin-2 (IL-2) receptor) and the transforming growth factor- (TGF ) receptor, and by the initiation of the cyclic AMP pathway together regulate the expression of the transcription factor forkhead box P3 (FOXP3). These events result in the activation of transcription factors that are involved in FOXP3 expression, including cAMP-responsive-element-binding protein (CREB), activating transcription factor (ATF), SP1, nuclear factor of activated T cells (NFAT), activator protein 1 (AP1), TGF -inducible early gene 1 (TIEG1), mothers against decapentaplegic homologue 3 (SMAD3) and signal transducer and activator of transcription 5 (STAT5). However, the contribution of each pathway (either beneficial or inhibitory) to FOXP3 expression might differ between different types of T cell; for example, CD28 stimulation is important for the thymic development of FOXP3+ regulatory T cells, whereas the activation of the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway inhibits FOXP3 expression in peripheral naive T cells by an unknown mechanism. ERK, extracellular-signal-regulated kinase; JAK, Janus kinase; JNK, JUN N-terminal kinase; PKA, protein kinase A; PKC , protein kinase C ; PLC , phospholipase C . http://www.nature.com/nri/journal/v9/n2/fig_tab/nri2474_F2.html
How does IL-21 work in enhancing Cytotoxic T-cell activity against cancer?
In vitro exposure to IL-21 (in contrast to other gamma-chain receptor cytokines) can lead to the generation of self-antigen–specific CTL in increased numbers and with enhanced avidity and function:
- IL-21 treatment alone led to a 10-fold decrease in the fraction of Foxp3+ cells (2.95% to 0.21%) in culture.
- The combination of CD25 depletion and IL-21 treatment resulted in a drop in the CD4+ Foxp3+ population to an almost undetectable level (2.95% to 0.017%); further, a substantial decrease in the fraction of CD4− Foxp3+ cells was observed that was not seen with either IL-21 treatment or CD25 depletion alone.
- The combination of CD25 depletion and IL-21 resulted in a more than 100-fold depletion of Foxp3+Tregs and augmented the frequency and absolute number of tumor antigen–specific CD8+ CTLs between 150- and 300-fold. This effect is much greater than that expected given the individual contribution of each factor and is unique for IL-21 since the addition of other γ-chain receptor cytokines such as IL-2, IL-7, and IL-15 did not have a similar effect.
- IL-21 also increased the expression of T-cells expressing CD28, which binds to CD80/B7 (expressed on T-helper cells) causing T-cell activation. IL-21 can reverse Treg-mediated suppression of cytoxic T-cells, as well.
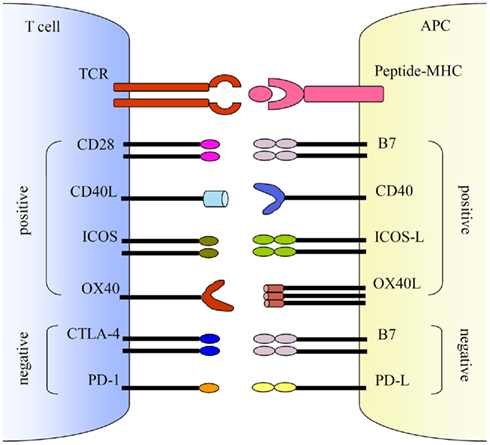
Co-stimulations either enhance or down-regulate T cell activation following the initial TCR and peptide-MHC ligation. Positive co-stimulatory pathways include B7–CD28, CD40L–CD40, ICOS–ICOS-L, and OX40–OX40L. Negative co-stimulatory pathways include B7–CTLA-4 and PD-1–PD-L. http://journal.frontiersin.org/article/10.3389/fmicb.2011.00202/full
In vitro treatment of autologous T-cells with IL-21 and CD25+ depletion is a promising strategy to remove the influence of Treg suppression and increase the activity of tumor-directed cytotoxic T-cells.

