Malignant tumors cannot grow beyond a certain size without establishing blood supply to feed them with necessary nutrients and oxygen. The process of recruiting new blood vessels to the tumor is termed angiogenesis. Growth factors, mainly secreted by the tumor itself, induce angiogenesis; the most notable of these is vascular endothelial growth factor (VEGF), which helps endothelial precursor cells mature into neo-capillary forming, endothelial cells. These secrete other growth factors, like platelet derivative growth factor (PDGF), which attracts pericytes and vascular smooth muscle cells that, together, create the outer layers of capillaries.
Tumor vasculature is haphazardly and chaotically arranged; only vessels on the periphery contain pericytes, which are functionally more stable than other cells of the tumor vasculature and are independent of VEGF for survival. In contrast, the majority of the tumor vasculature is not supported by pericytes and is tortuous, leaky and immature. And, the cells are dependent on VEGF and other growth factors for survival. The importance of angiogenesis in cancer progression makes it a good target for cancer therapy.
What are vascular disrupting agents (VDA) and how are they different from vascular targeting agents (VTA)?
VTAs inhibit the formation of new blood vessels, but do not act on already existing blood vessels, therefore, they prevent neo-vascularizationVDAs target endothelial cells and pericytes of the already established tumor vessels, leading to tumor ischemia and necrosis. VDA’s are more efficient in advanced disease and can be administered acutely. Although the mechanism of action of VDAs is not well understood, they destroy the endothelium of the solid tumors vasculature, thereby starving tumor cells of oxygen and other nutrients needed to thrive.
What is CA4P and how does it work?
Combretastatin A4-Phosphate (CA4P – prodrug of combretastatin A-4) comes from the family of chemicals that have been extracted originally from the “Combretum Caffrum,” the African willow tree. This is the most widely studied VDA and the first such agent to enter clinical trials; it is the lead product candidate of Oxigene, now Mateon Therapeutics.
It acts by reversibly binding to tubulin, thereby changing the endothelial cell structure; this ultimately leads to occlusion of tumor blood supply, which causes widespread ischemia and necrosis of tumor cells within the core of the tumor as shown in the Figures 2 and 3.
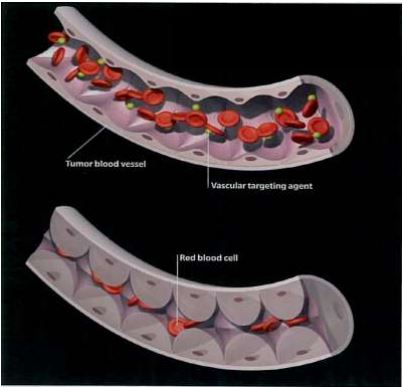
Figure 2: VDA’s directly binding to tubulin, reversibly, which changes endothelial cell structure leading to occlusion of blood vessels. Mateon Therapeutics
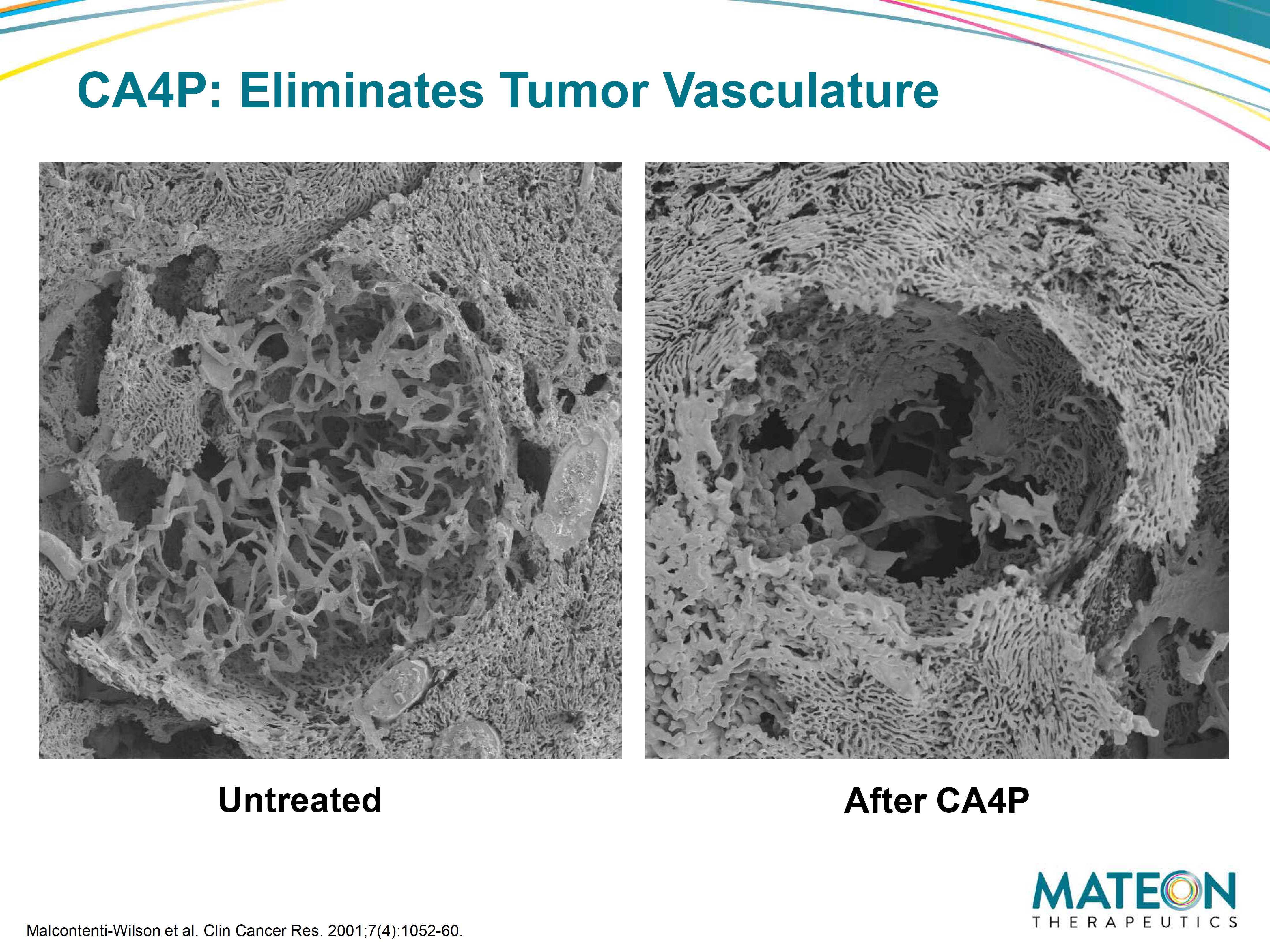
Figure 3: SEM (scanning electron microscope) of corrosion casts on liver metastasis. Untreated (left) figure shows dense tumor vessels at host-tumor interface. The right figure shows marked reduction of the patient’s tumor vasculature post treating with CA4P 100mg/kg. Mateon Therapeutics
Rationale for combining CA4P with classic anti-angiogenic therapy
Currently available classic anti-angiogenic drugs (VTAs) inhibit the formation of VEGF-driven new blood vessels. Avastin (bevacizumab) is the prototypic antiangiogenic drug – it is a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF). Since VEGF is the key angiogenic factor in tumors, blocking VEGF signal transduction can lead to tumor growth arrest and inhibition of metastasis. Avastin is the first antiangiogenic agent to demonstrate a survival benefit in patients with any type of cancer.
Although VDAs cause acute occlusion of existing vessels causing intra-tumor necrosis, CA4P treated tumors were left with a peripheral rim of viable tumor cells, which rely on surrounding blood vessels as seen in Fig 4. This residual viable rim of peripheral tumor cells allowed re-growth and re-vascularization of the tumor. It was proposed that VDA treatment led to an acute mobilization of circulating endothelial progenitor (CEP) cells, which home to the viable tumor rim, otherwise found in low numbers in the untreated cancer. For this reason Mateon therapeutics combined CA4P with bevacizumab. This combination of CA4P with VEGF inhibitors is the current focus of Mateon therapeutics.
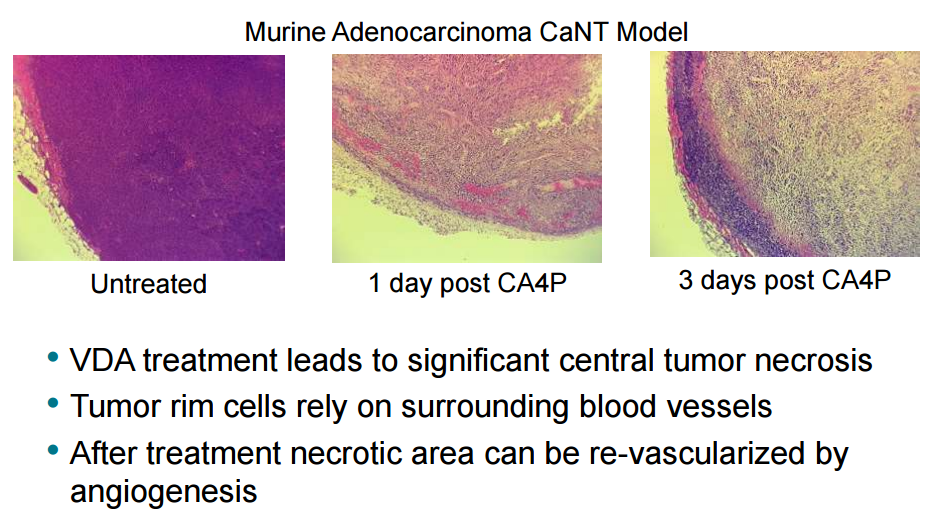
Figure 4: Hematoxylin and eosin staining of histological sections of murine adenocarcinoma CaNT tumors, pre and post treatment with 100mg/kg CA4P. The peripheral vascular rim of viable tumor cells are seen clearly 3 days post CA4P treatment. Mateon Therapeutics
Mateon therapeutics is exploring frosbretabulin’s activity in ovarian cancer, endocrine tumors, glioblastoma multiforme, gastric and hepatocellular carcinomas. The study carried out on patients with recurrent ovarian cancer demonstrated a statistically significant improvement in progression free survival (PFS) and a longer preliminary median overall survival (OS) in combined therapy compared to bevacizumab alone. Also, results indicated that the improvement in PFS was greater with combination therapy for patients having measurable disease, as opposed to those with non-measurable disease, suggesting that more established tumors (advanced disease) are particularly susceptible, which is consistent with the proposed mechanism of action. The only significant adverse event to be observed to date has been hypertension, which was easily treated with anti-hypertensive agents.
FOCUS, a phase II/III study of CA4P for the treatment of platinum resistant ovarian cancer, is currently underway; if successful, it will be used as the basis for a new drug application to the FDA.