In a phase II randomized trial, adding IMAB362 to standard chemotherapy increased progression-free survival (PFS) and overall survival (OS) by about 50% compared with the standard treatment alone.
What are Claudins?
IMAB362 selective and specific for the tight junction protein Claudin-18.2 (CLDN18.2). This unique target is responsible for regulating paracellular permeability and sealing the space between epithelial and endothelial cellular sheets. Its expression is restricted to differentiated stomach cells only and is absent from all other tested healthy tissues. The claudin18.2 molecule plays a role in cell adhesion and intercellular communication, but in tumors, claudin18.2 loses its primary role because the cancers disrupted the tight junctions between cells.
Claudins are integral membrane proteins and components of tight junction strands. Tight junction strands serve as a physical barrier to prevent solutes and water from passing freely through the paracellular space between epithelial or endothelial cell sheets, and also play critical roles in maintaining cell polarity and signal transductions. This gene is upregulated in patients with ulcerative colitis and highly overexpressed in infiltrating ductal adenocarcinomas. PKC/MAPK/AP-1 (protein kinase C/mitogen-activated protein kinase/activator protein-1) dependent pathway regulates the expression of this gene in gastric cells.
Isoform 2 of the tight junction molecule claudin-18 (CLDN18.2) is a highly selective cell lineage marker. Its expression in normal tissues is strictly confined to differentiated epithelial cells of the gastric mucosa, but it is absent from the gastric stem cell zone. CLDN18.2 is retained on malignant transformation and is expressed in a significant proportion of primary gastric cancers and the metastases thereof. It is a tumor associated transplantation antigen (TATA).
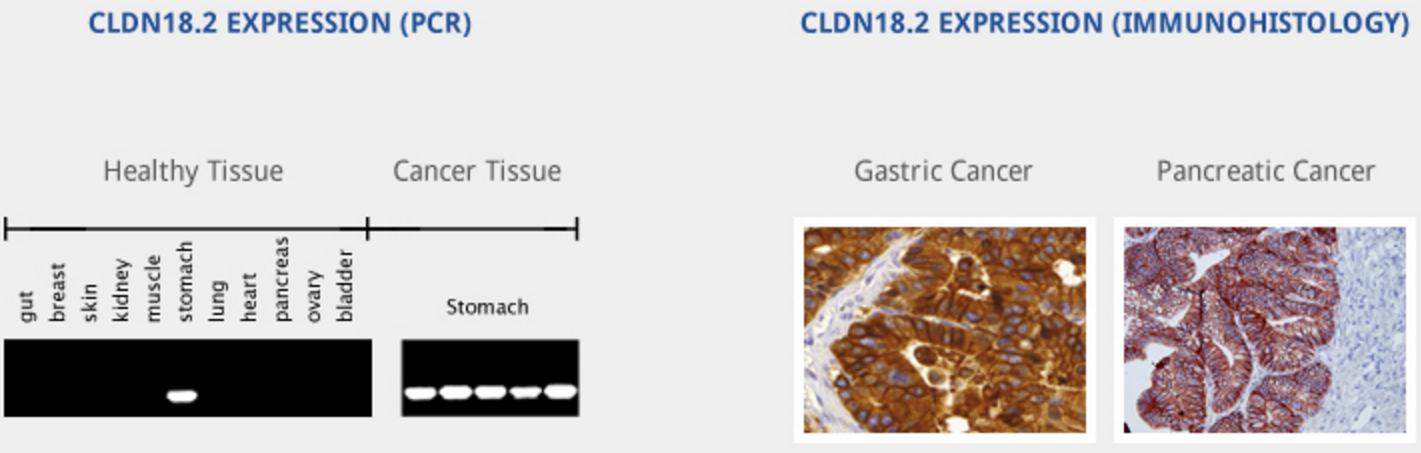
CLDN18.2 is overexpressed in up to 80% of gastrointestinal adenocarcinomas (primary and metastasized)1and 60% pancreatic tumors in addition to other solid cancers. http://www.ganymed-pharmaceuticals.com/pipeline/imab362.html
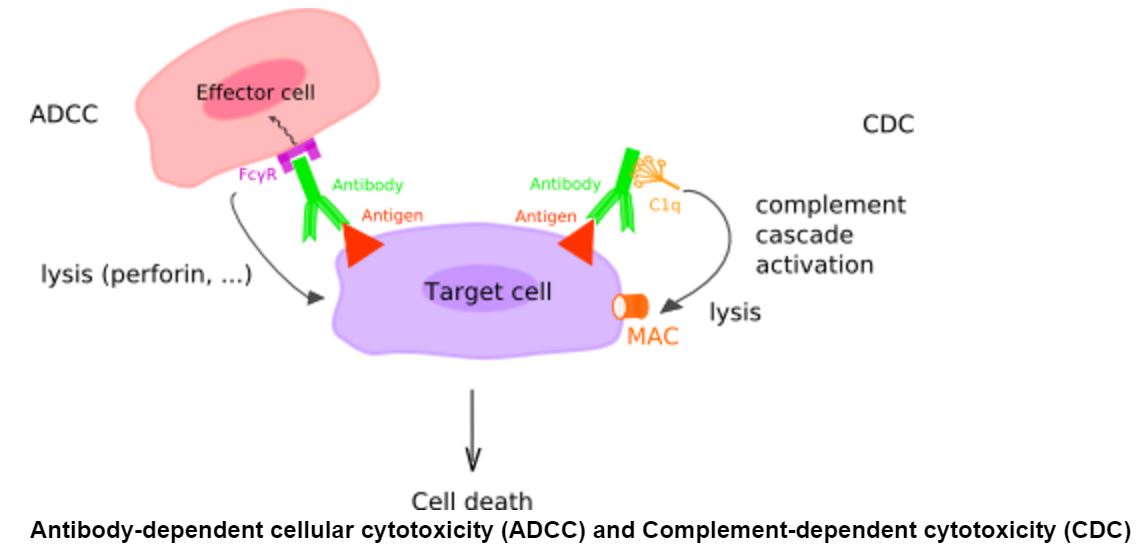
How does IMAB362 kill cancer cells?
When the antibody binds to tumor cells expressing the molecule, it causes antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) and kills the cell.
Clinical Trial in Advanced Gastric Cancer
Results of a Phase IIb (FAST) study showed that IMAB362 significantly extended median overall survival in patients with advanced gastric cancer when added to standard chemotherapy. Furthermore, IMAB362 nearly doubled survival in patients with the highest levels of Claudin18.2. The study included 161 patients with advanced or recurrent gastric or gastroesophageal junction cancer with a specific minimal level of Claudin18.2 in the tumor. Claudin18.2 levels were assessed from tumor biopsy specimen using the validated CE-marked diagnostic assay CLAUDETECT®18.2. Patients were then randomly assigned to receive standard chemotherapy [EOX – epirubicin, oxaliplatin and capecitabine (Xeloda)] with IMAB362 (800/600 mg/m2) or chemotherapy alone. Moreover, an additional 85 patients were treated in an added exploratory arm with a higher IMAB362 dose.
Compared to chemotherapy alone, IMAB362 extended the median time to disease progression from 4.8 to 7.9 months (HR 0.47, p=0.0001) and the median overall survival from 8.4 to 13.2 months (HR 0.51, p=0.0001). Among the patients with the highest levels of Claudin18.2 expression, the median overall survival was 16.7 months with IMAB362 and 9 months with chemotherapy alone (HR 0.45, p<0.0005).
The investigators also found patients getting the antibody were more likely to have a response: eight complete responses and 22 partial responses, compared with three and 18, respectively, in the control arm. On the other hand, the clinical benefit rate was 64% in both arms because of an increased proportion of stable disease in the control arm. Fewer patients getting the antibody had progressive disease (four) versus 10 patients receiving chemotherapy, alone.
Adverse events were more common in the IMAB362 group than in the control arm:
- Vomiting: 34.5% of patients with grade 1/ 2 and 3.6% with grade 3/4 in the control arm versus 55.8% of patients with grade 1/ 2 and in 10.4% with grade 3/4 in the IMAB362 arm;
- Bone marrow suppression: neutropenia: 21.4 % of patients with grade 1/ 2 and 21.4 % with grade 3/4 in the control arm versus 23.4% of patients with grade 1/ 2 and in 32.5% with grade 3/4 in the IMAB362 arm.