Antibody drug conjugate Glembatumumab vedotin (GV) is in late phase 2 trials for patients with triple negative breast cancer (TNBC) – those whose tumors do not express estrogen, progesterone, or HER-2. Approximately 15% of breast cancer patients have TNBC; it is an important area of research for both researchers and clinicians alike because:
- TNBC is a poor prognostic factor for disease-free and overall survival
- no effective specific targeted therapy is readily available for TNBC
- there is a clustering of TNBC cases in premenopausal women and in women of African descent
- the overlap of BRCA1-associated breast cancers with the TNBC phenotype is significant.
What is Glembatumumab vedotin (GV)?
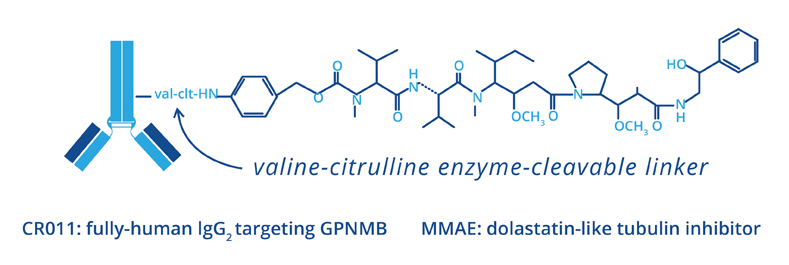
GV is a fully-human monoclonal antibody-drug conjugate (ADC) that targets glycoprotein NMB (gpNMB).
gpNMB is a protein overexpressed by multiple tumor types, including breast cancer and melanoma. gpNMB has been shown to be associated with the ability of the cancer cell to invade and metastasize and to correlate with reduced time to progression and survival in breast cancer. The gpNMB-targeting antibody, CR011, is linked to a potent cytotoxic tubulin inhibitor, monomethyl auristatin E (MMAE), using Seattle Genetics’ proprietary technology.
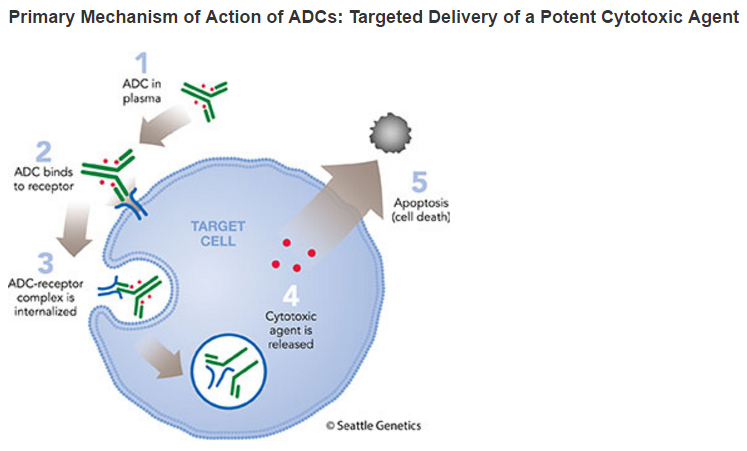
GV is designed to be stable in the bloodstream, but to release MMAE upon internalization into gpNMB-expressing tumor cells, resulting in a targeted cell-killing effect.
What is GPNMB?
gpNMB is a type I internalizable transmembrane protein; very little is known about its physiological function, but it is believed to be associated with cell invasion and motility particularly in breast cancer cells. Blockage of gpNMB induced not only HER2 but also EGFR expression. On the other hand, inhibition of HER2 by trastuzumab increased expression of gpNMB. Depletion of gpNMB increased sensitivity of trastuzumab, suggesting that gpNMB may play an important role in crosstalk of signal transduction for breast cancer. gpMNB is expressed in breast (5/6:83%), gastric (3/6:50%), colon (1/7:14.3%) cancer cell lines. Of breast cancer cell lines, gpNMB was highly expressed in SK-BR3 (HER2 positive), BT474 (HER2/ER positive), MDA-MB-157 (Triple negative) cells.
gpNMB is shed and measurable in the blood stream. Serum gpNMB in patients with breast (n = 164; primary 119, metastatic 43), gastric (n = 38), and colorectal (n = 50) were 9.403, 5.751, 6.550 ng/ml, respectively. gpNMB for breast cancer patients was statistically higher than those by colorectal cancer patients (p = 0.018). Of breast cancer patients, gpNMB for HER2-type patients was higher than those for Luminal type and DCIS patients (p = 0.0386, p = 0.0195, respectively). Those for triple negative patients was also higher than those for DCIS patients (p = 0.0459). Interestingly, serum gpNMB was dramatically reduced in accordance with chemotherapy in some patients.
gpNMB is a poor prognostic marker, associated with tumor invasion, metastasis, angiogenesis and reduced time to progression and survival. Approximately 40% of triple-negative breast cancer show over-expression of gpNMB.
Initial Clinical Trial Results
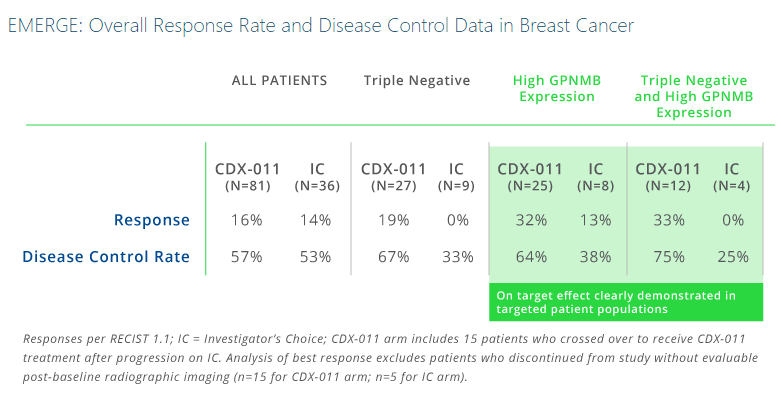
In the EMERGE Phase 2 trial, treatment of patients with both TNBC and over-expression of gpNMB showed a high overall response rate (ORR) of 33% when treated with glembatumumab vedotin. In comparison, these researchers found no responses in patients with both TNBC and over-expression of gpNMB treated with standard chemotherapies.
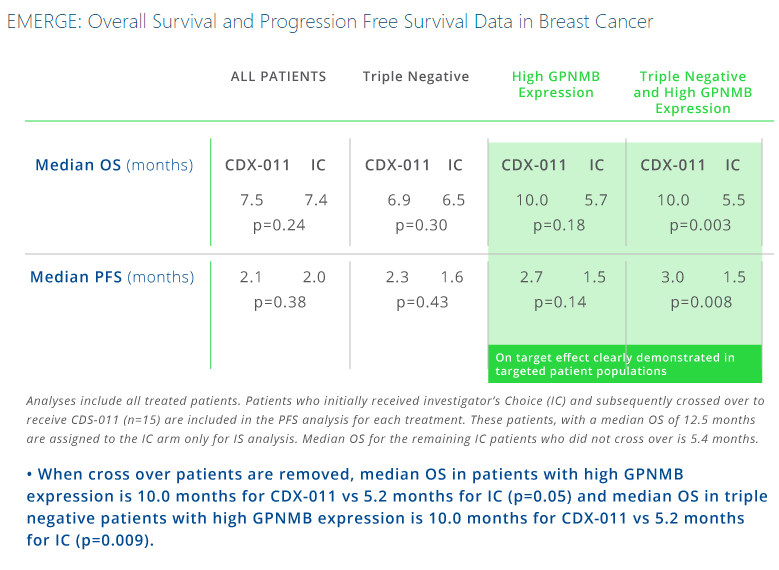
In additional subset analyses of the EMERGE trial, objective response rate was 30% (7/23) for glembatumumab vedotin vs. 9% (1/11) for investigator’s choice in tumors with GPNMB-overexpression (>25% of tumor epithelium); 18% (5/28) vs. 0% (0/11) in TNBC; and 40% (4/10) vs. 0% (0/6) in gpNMB-overexpressing TNBC for glembatumumab vedotin and IC respectively. This trial also showed apparent improvements in progression-free survival (PFS; hazard ratio (HR) = 0.11) and overall survival (OS; HR = 0.14).
Future Clinical Trials
The METRIC Trial (NCT01997333) is an international, two-arm phase II study in which patients are randomized 2:1 to GV (1.88 mg/kg IV q 21 days) or capecitabine, a current standard of care for this population (2,500 mg/m2 daily for d1-14, q21 days) until progression or intolerance. Crossover in trial is not permitted.
To be eligibility to participate in this trial, patients must meet criteria including >25% of tumor epithelium gpNMB+ by central immunohistochemistry (IHC) screening of archival tissue; estrogen receptor and progesterone receptor <10% and HER2 negative [0-1+ IHC, or ISH copy number <4.0/ratio <2.0] by local assessment; ECOG 0-1; taxane resistance; anthracycline exposure (if indicated); <2 chemotherapy regimens for advanced BC; measurable disease; no persistent Grade >2 toxicity.
The trial has 85% power to detect a PFS (progression-free survival) HR (hazard ratio) of 0.64 with two sided α = 0.05. The hypothesized median progression-free survival is 4.0 months for capecitabine and 6.25 months for GV. Target accrual is open for 300 patients. While this is a Phase 2 study, the patient size is large enough such that it could be submitted for FDA approval if the evidence is strong enough.
Additional studies in advanced melanoma, osteosarcoma, and lung cancer are ongoing.