Halaven (eribulin mesylate) was approved by the FDA for the treatment of patients with unresectable or advanced liposarcoma. The treatment is approved for patients who previously had undergone chemotherapy with an anthracycline drug. It was previously approved for patients with breast cancer who had undergone at least two prior chemotherapeutic regimens, including an anthracycline and a taxane.
HALAVEN contains eribulin mesylate, a microtubule dynamics inhibitor. Eribulin mesylate is a synthetic analogue of halichondrin B, a product isolated from the marine sponge Halichondria okadai. Eribulin inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates. Eribulin exerts its effects via a tubulin-based antimitotic mechanism leading to G2/M cell-cycle block, disruption of mitotic spindles, and, ultimately, apoptotic cell death after prolonged mitotic blockage.
Microtubule inhibitors such as taxanes (e.g., Taxol – paclitaxel, Taxotere – docetaxel, and Abraxane – albumin-bound paclitaxel) and vinca alkaloids (e.g., Vinblastine and Oncovin – vincristine) have been reported to be highly effective against breast cancer as well as many other types of cancer. Similar to these drugs, eribulin is a microtubule inhibitor that induces apoptosis of cancer cells by stopping mitosis in the G2/M phase of the cell cycle.

Two MTD-induced cell cycle checkpoints.
This illustration shows the proposed relative location of the 2 known cell cycle arrest points in cells with MTD (microtubule damage). SAC indicates spindle assembly checkpoint; G1MTC, G1 phase microtubule checkpoint. The centrosome cycle (1 or 2 MTOC indicates microtubule organizing centers) is shown to be coordinated with the DNA/chromosome cycle (2N or 4N DNA content). The arrests the cell cycle in G1 phase between the shift from a low cdc2-expressing state to a high cdc2-expressing state. http://www.bloodjournal.org/content/97/5/1505?sso-checked=true
Recent studies have demonstrated that the mode of inhibitory action of eribulin differs from mechanisms of conventional microtubule inhibitors:
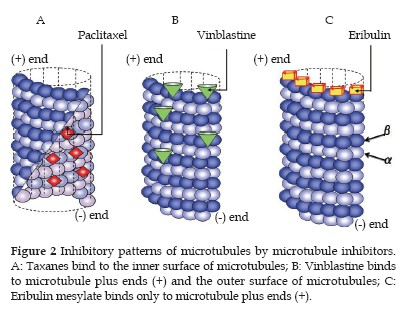
- Taxanes (paclitaxel and docetaxel) bind to the inner surface of microtubules and promote microtubule lengthening (polymerization), while inhibiting microtubule shortening (depolymerization).
- Vinca alkaloids (vincristine, vinblastine, etc.) bind to microtubule plus ends and the outer surface of microtubules, inhibiting microtubule lengthening (polymerization) as well as shortening (depolymerization) (Figure 2B).
- In contrast, eribulin binds to only microtubule plus ends and inhibits only microtubule lengthening (polymerization), without affecting shortening (depolymerization).
Because eribulin selectively binds with highly affinity to only microtubule plus ends, a small number of molecules can exert antitumor effect through microtubule inhibition. Binding of one molecule of eribulin to two microtubules can inhibit cell proliferation by 50%, and such binding is reversible. A recent analysis showed that eribulin binds to a site near the guanosine 5′-triphosphate (GTP) binding site. Because eribulin strongly binds only to this unique site involved in microtubule polymerization, eribulin produces antitumor activity at much lower drug concentrations than conventional microtubule inhibitors.
According to the FDA, approximately 1000 people all over the world are diagnosed each year with liposarcoma. Moreover, it would seem that this type of cancer has a high death rate. But, according to the FDA, this new drug is capable of extending the patient’s life by 15 months, compared to the old drug, dacarbazine (a DNA alkylating agent), which had a median overall survival of 8.4 months.
In one study reported in May 2015, 452 patients (67% female; 79% younger than age 65 years) with liposarcoma and leiomyosarcoma were included. Researchers randomly assigned 228 patients to 1.4 mg/m² eribulin intravenously on days 1 and 8 every 21 days and 224 patients to 850 mg/m² to 1200 mg/m² dacarbazine intravenously on day 1 every 21 days. Patients received treatment until disease progression.
- OS (overall survival) served as the study’s primary endpoint and median PFS (progression-free survival), 12- week PFS and safety served as secondary endpoints. Median OS was 13.5 months in the eribulin group compared with 11.5 months in the dacarbazine group (HR = 0.77; 95% CI, 0.62-0.95).
- PFS was 2.6 months in both treatment cohorts (HR = 0.88; 95% CI, 0.71-1.09). Thirty-three percent of patients who received eribulin achieved 12-week PFS and compared with 29% of patients assigned dacarbazine.
- More patients in the eribulin arm required dose reductions (26% vs. 14%). Further, 8% of the patients assigned eribulin and 5% of the patients assigned dacarbazine discontinued treatment due to a drug-related adverse event.
- The most common adverse events associated with eribulin included neutropenia (44%), pyrexia (28%), peripheral sensory neuropathy (20%) and alopecia (35%). Of these adverse events, 63% were grade 3, 26% were grade 4 and 4% were fatal.
- Adverse events occurred less frequently in the dacarbazine arm; however, thrombocytopenia occurred in more patients treated with dacarbazine than eribulin (29% vs. 6%).
The FDA cited a subgroup from this trial including only 143 patients with liposarcoma. In these patients, the median overall survival was 15.6 months with eribulin and only 8.4 months with dacarbazine.
Yondelis (trabectedin) is an alkylating agent that has been shown to have a superior progression-free interval compared to dacarbazine in patients with liposarcoma and leiomyosacrcoma:
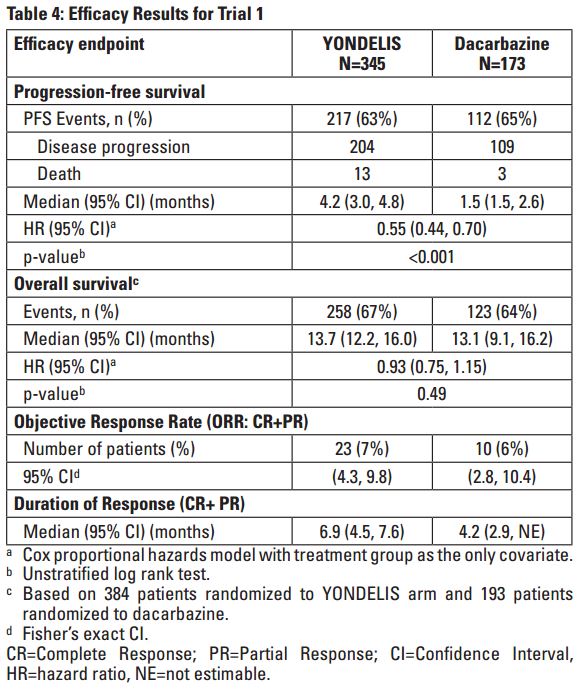
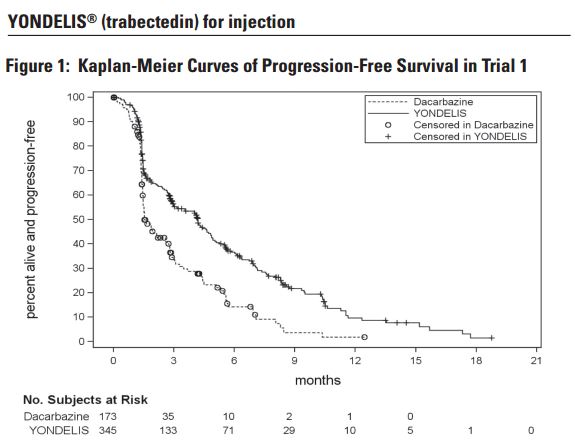
- The clinical efficacy and safety of YONDELIS in patients with metastatic or recurrent leiomyosarcoma or liposarcoma were demonstrated in Trial 1, a randomized (2:1), open-label, active-controlled trial comparing treatment with YONDELIS 1.5 mg/m2 as a 24-hour continuous intravenous infusion once every 3 weeks to dacarbazine 1000 mg/m2 intravenous infusion (20 to 120 minutes) once every 3 weeks. Treatment continued in both arms until disease progression or unacceptable toxicity; all patients in the YONDELIS arm were required to receive dexamethasone 20 mg intravenous injection prior to each YONDELIS infusion.
- Patients were required to have unresectable, locally advanced or metastatic leiomyosarcoma or liposarcoma (dedifferentiated, myxoid round cell, or pleomorphic) and previous treatment with an anthracycline- and ifosfamide-containing regimen or an anthracycline-containing regimen and one additional cytotoxic chemotherapy regimen. Randomization was stratified by subtype of soft tissue sarcoma (leiomyosarcoma vs. liposarcoma), ECOG performance status (0 vs. 1), and number of prior chemotherapy regimens (1 vs. ≥2).
- The efficacy outcome measures were investigator-assessed progression-free survival (PFS) according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1), overall survival (OS), objective response rate (ORR), and duration of response (DOR). A total of 518 patients were randomized, 345 to the YONDELIS arm and 173 patients to the dacarbazine arm. The median patient age was 56 years (range: 17 to 81); 30% were male; 76% White, 12% Black, and 4% Asian; 73% had leiomyosarcomas and 27% liposarcomas; 49% had an ECOG PS of 0; and 89% received ≥2 prior chemotherapy regimens. The most common (≥20%) pre-study chemotherapeutic agents administered were doxorubicin (90%), gemcitabine (81%), docetaxel (74%), and ifosfamide (59%). Approximately 10% of patients had received pazopanib.
- Trial 1 demonstrated a statistically significant improvement in PFS – 4.2 months versis 1.5 months for Yondelis and dacarbazine, respectfully (P < 0.001).