The National Cancer Institute launched the MATCH trial in August 2015 as part of the Precision Medicine Initiative announced by President Obama.
The FDA approaches cancer drug development from the perspective of tumor type as defined initially by site of origin, and secondarily by genetic mutation. On the other hand, the NCI views cancer as a disease of mutations, and categorizes them according to the affected genes, irrespective of the tissue of origin. For example, if both a breast cancer and gastric cancer over-express the EGFR (epidermal growth factor receptor), the NCI considers these tumors as a single entity in the practice of precision medicine.
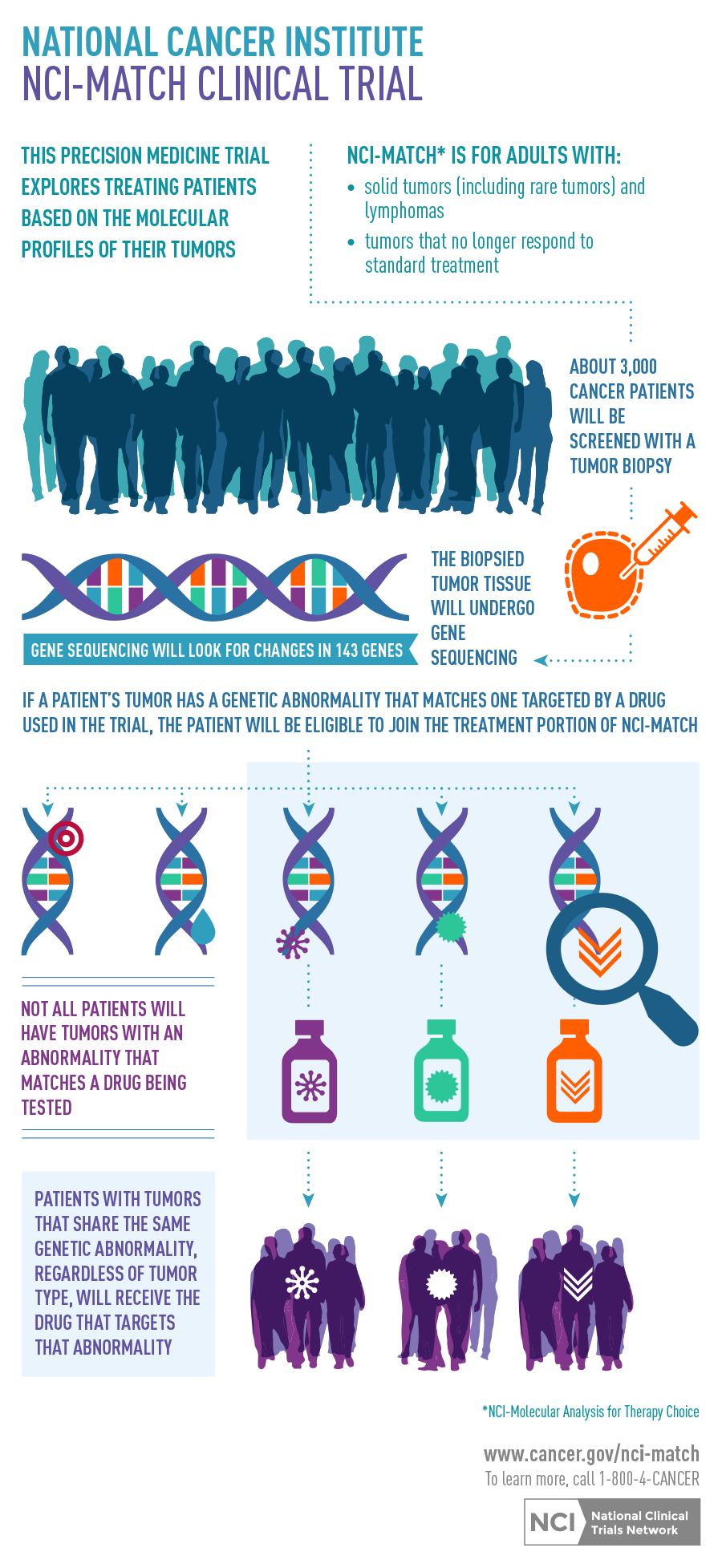
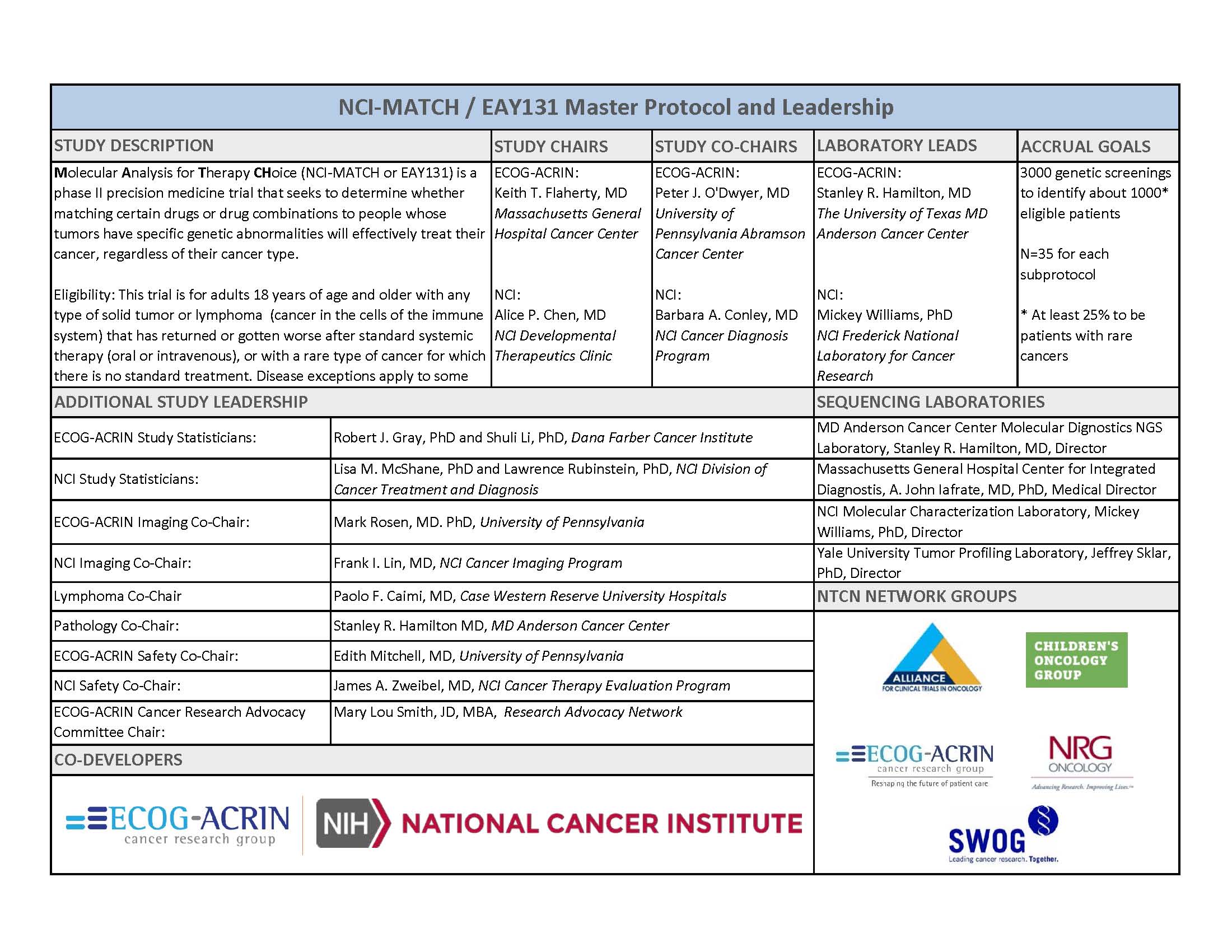
The National Cancer Institute-Molecular Analysis for Therapy Choice, known as NCI-MATCH or study EAY131, is a $30 million phase II precision medicine trial that seeks to determine whether matching certain drugs or drug combinations in adults whose tumors have specific gene abnormalities will effectively treat their cancer, regardless of their cancer type.
Precision medicine refers to the tailoring of treatment based on the characteristics of each individual. Treatment focuses on molecular abnormalities instead of the organ site of the cancer.
- NCI-MATCH is a unique cancer treatment trial because it is the largest, most scientifically rigorous precision medicinecancer trial to date
- NCI-MATCH is considered the largestprecision medicine cancer trial to date based on the number of patients, treatment options, and types of cancer being studied in a single clinical trial
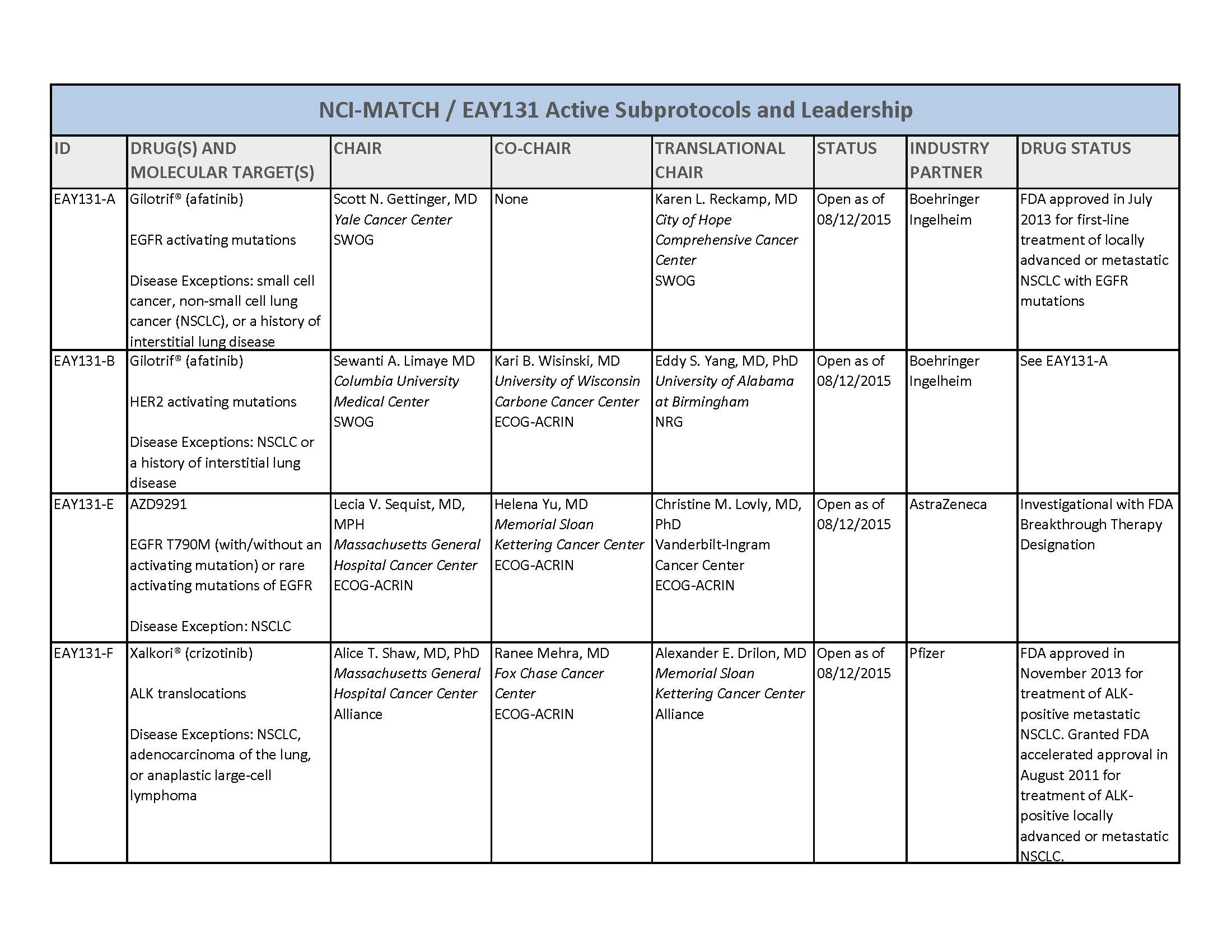
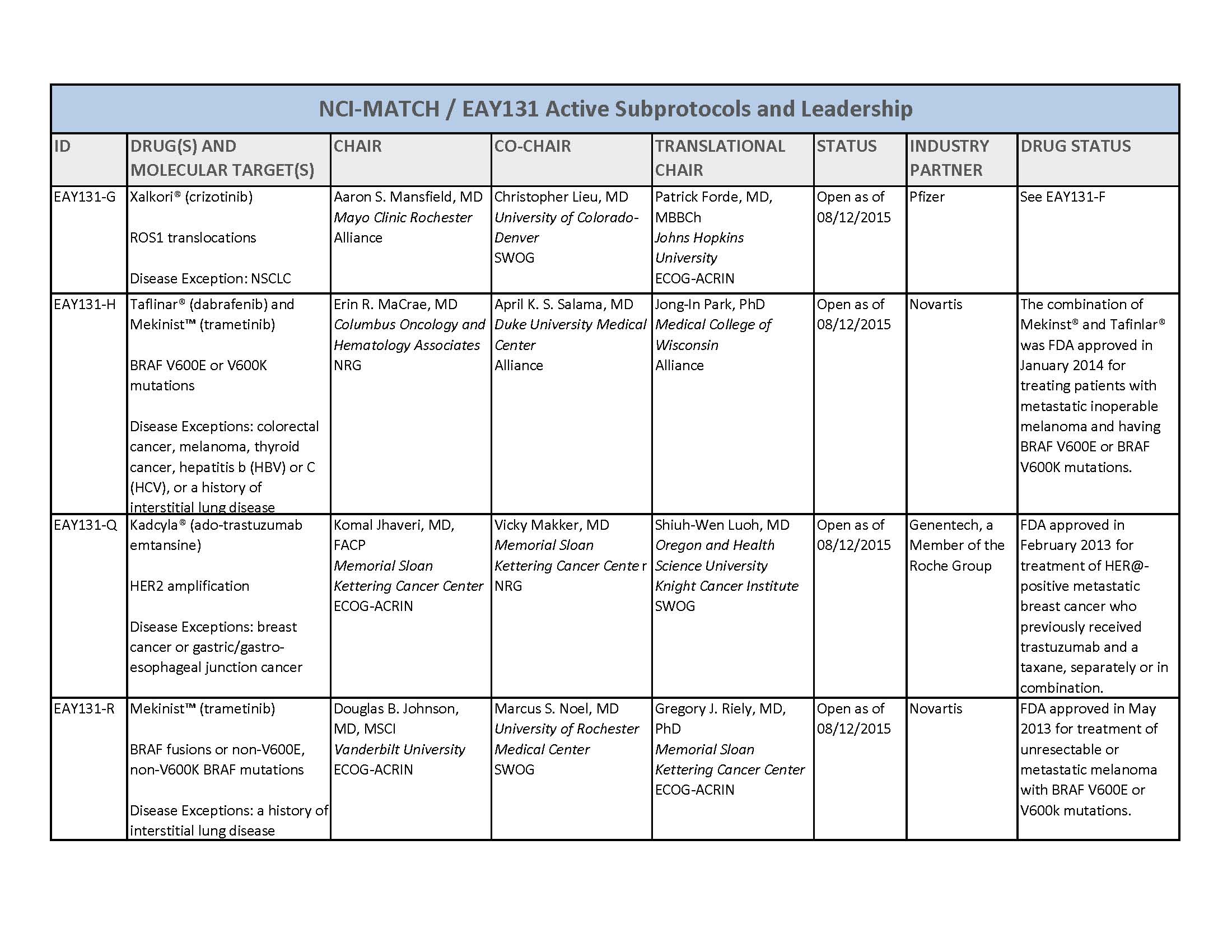
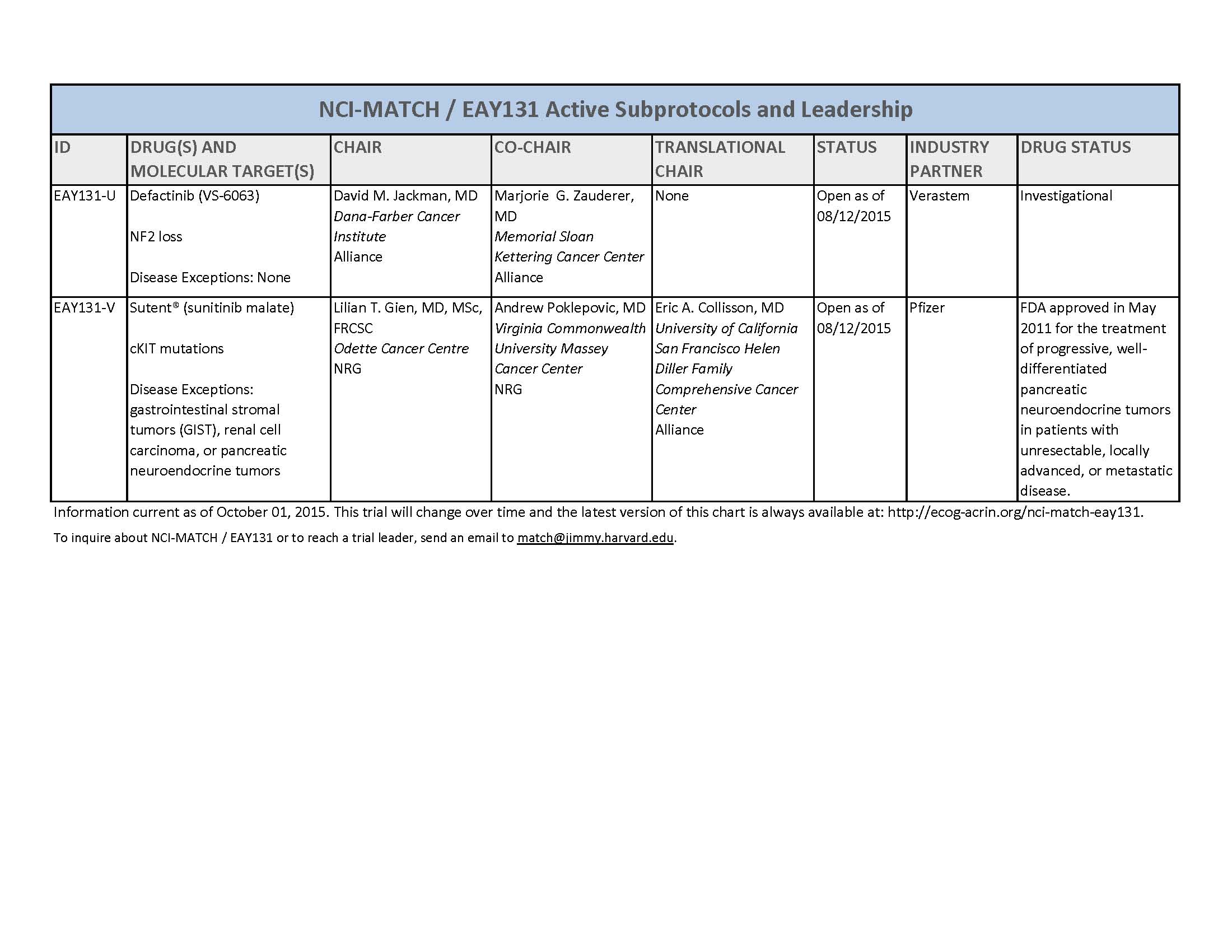
- About 3000 patients will receive genetic testing to identify those eligible for one of 22 or more treatment options
- The 22 or more treatment options explore a large number of genetic abnormalities and drugs—far more than any other cancer trial
- It includes a large number of cancer types (most other trials address a single cancer) and has many more drugs available than most other cancer trials
- A network of four molecular diagnostics laboratories provides capacity for the large number of patients
- NCI-MATCH is considered the most scientifically rigorousprecision medicine cancer trial to date
- A single DNA sequencing test was especially developed for this trial to look for 143 genes associated with cancer
- The use of a unique kit for biospecimen collection, shipment, and centralized processing assures high-quality analysis
- Cutting edge molecular analysis will be performed by four laboratories. All four labs demonstrated consistent results in pilot testing—an important aspect of quality assurance in a trial of this scope and scale.
Patient Eligibility
- The trial is for adults 18 years of age and older with any type of solid tumor or lymphoma (cancer in the cells of the immune system) that has returned or gotten worse after standard systemic therapy (oral or intravenous)
- Patients may also be eligible if they have a rare type of cancer for which there is no standard treatment
- The trial’s design calls for at least 25 percent of patient enrollment to be people with rare types of cancer
Patient Selection (Enrollment)
- About 3000 patients who meet these eligibility requirements will enroll in the initial screening step of the trial and have a new biopsy
- The biopsy samples will undergo genetic testing to identify molecular abnormalities
- If a molecular abnormality is detected that is targeted by one of the drugs being studied in the trial, patients will undergo further evaluation to determine if they meet the specific eligibility requirements of that treatment arm and can be accepted in the trial
Treatment Overview
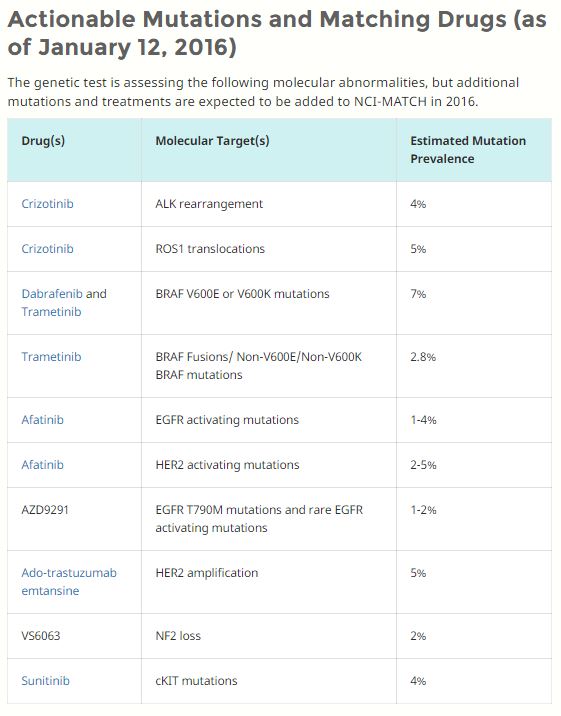
- Trial researchers expect that only a small number of those screened will have one or more molecular abnormalities that match one of the 22 or more treatment options being studied
- Once enrolled, patients will be treated with a drug or drug combination that targets the molecular changes in their tumor for as long as their tumor shrinks or remains stable
- The drugs included in the trial have all either been approved by the U.S. Food and Drug Administration (FDA) for specific cancer types or are still being tested in other clinical trials but have shown some effectiveness against tumors with a particular genetic alteration(s)
The study was paused in November so that the trial leaders could assess how it was progressing. Dr. Keith Flaherty, the oncologist who is leading the trial, didn’t expect the interim analysis to reveal such stark and frequent quality concerns in the biopsy samples. The issues include low volume of sample (the trial protocol calls for four biopsy cores, but routinely only two cores are being received by the central laboratory) as well as the quality of the biopsy specimens (cores containing very few cancer cells).
Overall, 10 to 20 percent of the samples did not yield enough cancer tissue for DNA testing, Flaherty said. More often than not, he found, these subpar biopsies came from smaller, more rural treatment centers, though many did come from major academic research hospitals.
The slipshod biopsies had real implications for the patients.
To be eligible to enroll in the MATCH trial, cancer patients had to have exhausted all standard treatment options. So this trial was often their last chance.
But the bad biopsies meant they couldn’t be matched with a personalized therapy. With time ticking, they grasped at straws. Many tried more chemotherapy, usually in vain. Others scrambled to find other clinical studies testing experimental therapies. Some simply entered hospice care.
Biopsy quality represents a real paradox – traditionally, it is advisable to make a diagnosis by removing as little tissue as possible from the patient by doing so with minimally invasive techniques. However, for this trial, which hinges upon genetic analyses, more tissue is better. Also, unlike routine clinical practice, patients must be biopsied upon every relapse so that genetic analyses can reveal the mutations that induced resistance.
Not only must this issue be resolved for the NCI MATCH trial, but also in routine medical practice in the future, which is evolving to molecular-based medical decision-making.
The MATCH Trial is being run by the ECOG-ACRIN Cancer Research Group is a multidisciplinary, membership-based scientific organization that designs and conducts biomarker-driven cancer research involving adults who have or are at risk of developing cancer. The Group is dedicated to its stated purpose, which is to achieve research advances in all aspects of cancer care and thereby reduce the burden of cancer and improve the quality of life and survival in patients with cancer.
The Group was formed in May 2012 by a merger that combined the complemen tary strengths of the Eastern Cooperative Oncology Group (ECOG) in cancer therapy and the American College of Radiology Imaging Network (ACRIN) in cancer imaging. ECOG and ACRIN were two highly respected National Cancer Institute-sponsored cancer cooperative groups. ECOG’s large-scale cancer treatment clinical trials changed the standard of care in numerous types of cancer and helped to individualize cancer therapy. ACRIN’s clinical trials encompassed the full range of medical imaging research, including the investigation of surveillance strategies in high-risk populations, imaging biomarkers in early phase trials, prevention approaches in landmark cancer screening trials, and methodologies in comparative-effectiveness research.


Good luck on Friday. You should talk to your doctor about all of this – the biopsy procedure, as well as therapeutic options and their side effects. You have every right to have your questions answered – don’t be afraid to ask and to engage your doctor in a discussion about these topics.
I am scheduled for a biopsy this coming Friday 10/28. Will they take a specimen from more then one tumor? Will they take four specimens from each per your article above.live in Columbus Oh , Riverside Hospital. What are the side effects from the drugs should I be accepted?