An independent data monitoring committee (IDMC) has recommended the continuation of Argos’ pivotal phase 3 ADAPT clinical trial of AGS-003 for the treatment of metastatic renal cell carcinoma (mRCC) based on results of the committee’s second planned interim data analysis. The next planned interim analysis will be in 6 months.
The ADAPT trial is a Phase 3 study in 450 patients with newly diagnosed metastatic renal cell carcinoma who are randomized to receive standard therapy [Sutent – sunitinib, an inhibitor of multiple receptor tyrosine kinases including platelet-derived growth factor (PDGFR), vascular endothelial growth factor (VEGFR), FLT3, stem cell receptor facor (KIT), neurotrophic factor (RET), and colony stimulating factor (CSF-1R)] or standard therapy plus AGS-003. The primary endpoint of the study is survival and secondary endpoints include progression-free survival and tumor response.
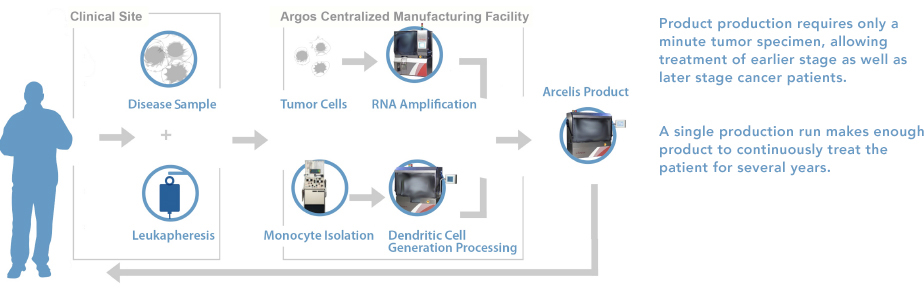
AGS-003 is administered via intradermal injection, with 8 injections in the 1st year followed by quarterly boosters. It is an autologous dendritic cell (DC) vaccine. Whole tumor RNA from each individual patient (whether from the primary tumor site, distant metastatic sites or from tumor cells present in circulation) are isolated amplified. Then, the tumor mRNA is transfected into the same patient´s dendritic cells.
To create AGS-003, ribonucleic acid (RNA) is isolated from a small tumor sample obtained from standard tumor removal surgery (nephrectomy) or surgical biopsy (metastasectomy), and the patient´s dendritic cells are taken during a single leukapheresis procedure. The tumor RNA is used to “program” the dendritic cells with the entire disease-antigen repertoire to trigger an immune response against the patient´s specific cancer.
AGS-003 is prepared ex vivo from matured monocyte-derived DCs co-electroporated with the patient’s amplified tumor RNA and synthetic CD40L. When administered by intradermal injection, these optimized, RNA-loaded mature DCs are capable of presenting the relevant patient-specific tumor antigens, via MHC-Class I presentation, to T cells in the draining lymph node basin. CD40L precipitates CD4+ Helper T-cell interactions, which license the DC’s to engage CD8+ cells. Additionally, CD40 ligation optimizes CD8+ T cell induction through production of IL-12.
Importantly, the role of dendritic cells is to present exogenous antigens (phagocytosed proteins from cancer cells) in the context of MHC Class II. Cancer cells, themselves, present their antigens to the immune system in the context of MHC Class I. However, almost all tumors evade an immune response through multiple mechanisms, including the down-regulation of MHC class I and tumor-specific antigens, establishment of an immunosuppressive microenvironment, and subsequent hypo-responsiveness of antitumor effector cells. One of the most common means by which tumors evade the host immune response is by down-regulation of MHC class I molecule expression. Argos’ approach allows for dendritic cells, which are professional antigen presenting cells that produce immunologically active cytokines and possess important accessory molecules, to present antigens on their MHC Class I molecules. This allows for much more efficient cancer antigen presentation (by dendritic cells) and could precipitate a potent immune response and overcome immunological tolerance.

Multiple tumour necrosis factor (TNF) superfamily ligands and receptors sequentially participate in T cell–dendritic cell (DC) crosstalk. The events that occur following the activation of antigen-bearing DCs are outlined in the figure. DCs are activated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and consequently upregulate their expression of CD40, MHC molecules and the co-stimulatory molecules CD80 and CD86. The presentation of peptide antigens in the context of MHC class II molecules to antigen-specific CD4+ T cells, together with co-stimulatory signals (from CD80 and/or CD86), results in the activation of CD4+ T cells and the upregulation of the DC licensing factors CD40 ligand (CD40L) and lymphotoxin-α1β2(LTα1β2). Expression of CD40L and LTα1β2 on activated antigen-specific CD4+ T cells induces signalling through CD40 and the LTβ receptor (LTβR), and this licenses DCs. CD40 signalling results in the production of interleukin-12 (IL-12) and the upregulation of CD70, CD86, 4-1BB ligand (4-1BBL), OX40 ligand (OX40L) and GITR ligand (GITRL), whereas LTβR signalling leads to the production of type I interferons (IFNs). PAMPs and DAMPs also contribute to these events. Priming of CD8+ T cells by MHC class I-restricted peptides results in the upregulation of CD27, 4-1BB, OX40 and GITR (glucocorticoid-induced TNFR-related protein). Stimulation of these receptors on CD8+ T cells by their cognate TNF superfamily ligands, in combination with IL-12 and type I IFNs, results in robust CD8+ T cell activation, proliferation and effector function, as well as the formation and maintenance of CD8+ T cell memory. PRR, pattern-recognition receptor; TCR, T cell receptor. http://www.nature.com/nri/journal/v12/n5/fig_tab/nri3193_F1.html
In a multicenter Phase 2 trial, the median overall survival of 30.2 months was observed for all patients treated with the combination of AGS-003 plus sunitinib. According to the International mRCC Database Consortium, similar unfavorable risk mRCC patients have an expected overall survival of 14.7 months. In addition, in the Phase 2 trial, patients with intermediate risk experienced a median survival in excess of 5 years, while the expected median survival for intermediate risk mRCC patients treated with sunitinib as their first line targeted therapy is approximately 20.5 months.
“In comparison to the benchmarks established for similar risk mRCC patients treated with targeted therapy, the outcomes in this study were encouraging,” said principal investigator, Dr. Amin. “Mature data from this Phase 2 study suggest the combination of AGS-003 plus sunitinib was safe, well tolerated and associated with doubling of the expected median survival and encouraging long-term and 5-year survival.”
“AGS-003 is designed to generate a patient and tumor-specific immune response,” said study co-author Dr. Robert Figlin. “Observations from this study indicate that an increase in memory T-cells after five doses of AGS-003 was associated with prolonged survival. These important findings are being further evaluated in the ongoing Phase 3 ADAPT study.”



This is an interesting technique to use for cancer treatment and after reading this post I believe it may have a positive outcome. I do wonder how long the quarterly booster shots would be needed in order ensure that the cancer does not return. Therefore, I am interested in how long the booster shots would be administered for and would the cancer cells have a high chance of recurrence if the booster shots were not performed?