The best way to cure cancer is to catch it early when surgical removal of a localized mass can successfully rid the patient of all cancer cells and cancer stem cells. This is no more important than in pancreatic cancer for which late stage therapies have not been shown to be very effective. (Thank you, Elaine, for sending me this article to discuss on the blog.)
Pancreatic Cancer Statistics
An estimated 48,960 new cases of pancreatic cancer are expected to occur in the US in 2015. An estimated 40,560 deaths are expected to occur in 2015, about the same number in women (19,850) as in men (20,710).
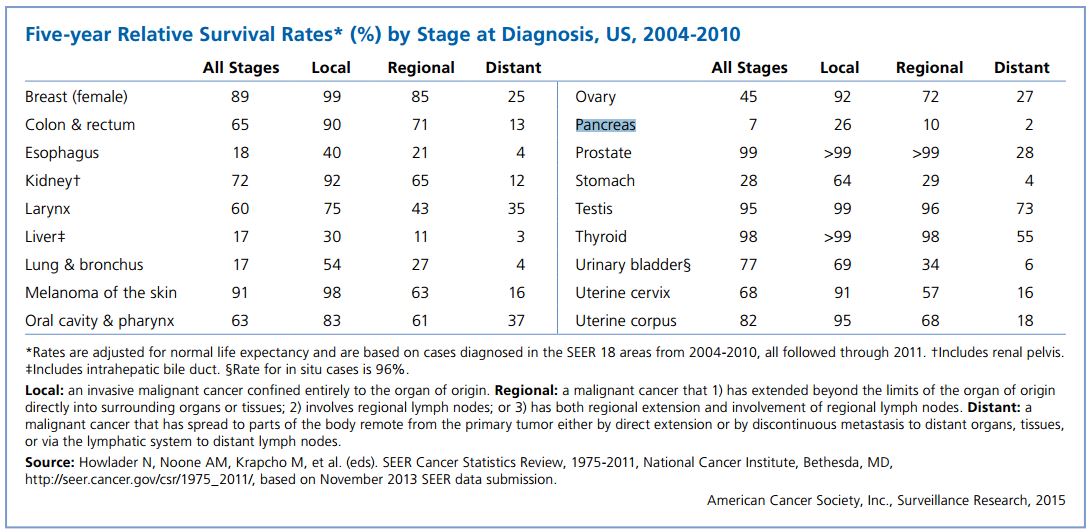
For all stages combined, the 1- and 5-year relative survival rates are 28% and 7%, respectively. This means that 72% of patients diagnosed with pancreatic cancer die within 12 months; for patients diagnosed with distant metastases, the 1- and 5-year survival is just 15% and 2%, respectively. Pancreatic cancer has the lowest survival rates of all cancers and is the third leading cause of cancer mortality (40,560 pts), trailing only lung cancer (158,040 pts) and colorectal cancer (49,700 pts).
New way to Detect Pancreatic Cancer
Melo and colleagues identified a cell surface proteoglycan, glypican-1 (GPC1), specifically enriched on cancer-cell-derived exosomes. GPC1+ circulating exosomes (crExos) were monitored and isolated using flow cytometry from the serum of patients and mice with cancer. GPC1+ crExos were detected in the serum of patients with pancreatic cancer (n = 56) with absolute (meaning 100%) specificity and sensitivity, distinguishing healthy subjects (n = 20) and patients with a benign pancreatic disease (n = 6) from patients with early- and late-stage pancreatic cancer. Levels of GPC1+ crExos correlate with tumour burden and the survival of pre- and post-surgical patients. GPC1+ crExos from patients and from mice with spontaneous pancreatic tumours carry specific KRAS mutations, and reliably detect pancreatic intraepithelial lesions in mice despite negative signals by magnetic resonance imaging. GPC1+ crExos may serve as a potential non-invasive diagnostic and screening tool to detect early stages of pancreatic cancer to facilitate possible curative surgical therapy.
What are Exosomes?
Exosomes are small (30–90 nm) extracellular vesicles derived from the multivesicular body (MVB) sorting pathway. These vesicles are produced by a wide variety of cell types including reticulocytes, epithelial cells, neurons, and tumor cells (Thery et al., 2009). Exosomes have been isolated from bronchoalveolar lavage, urine, serum, bile, and breast milk (Thery, 2011). Although previously considered to be cellular waste products (Thery, 2011), recent studies have demonstrated that exosomes are “bioactive vesicles” that promote intercellular communication and immunoregulatory processes by shuttling molecules between cells (Thery et al., 2009; Pegtel et al., 2010). Exosomes are lipid-bilayer-enclosed extracellular vesicles that contain proteins and nucleic acids, including miRNA’s (inhibitory RNA sequences).
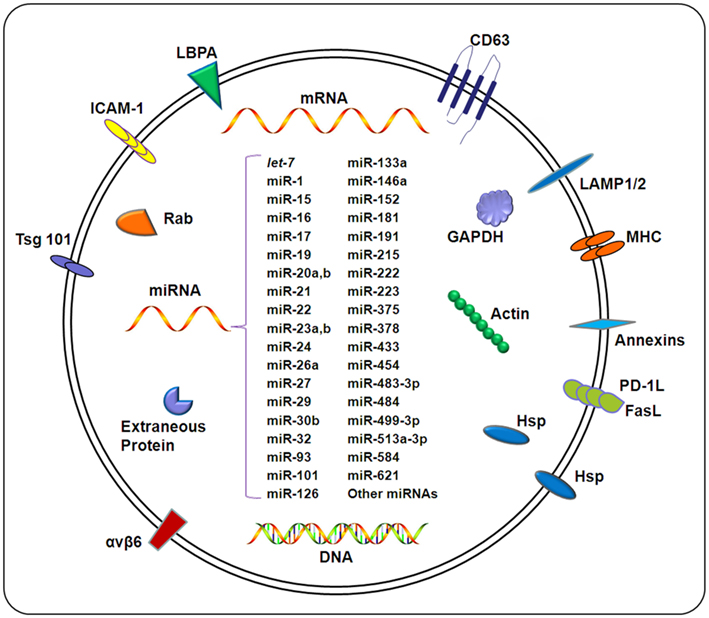
Composition of exosomes. Exosomes carry a wide array of molecules including proteins, DNAs, mRNAs, and miRNAs, depending on a variety of factors including the cell type from which the exosome originates, the state of health of the host, and extracellular stimuli. The contents of exosomes can be transferred from origin cells to target cells, resulting in an elaborate intercellular communication network. Exosomal miRNAs confirmed by different methods (e.g., microarray and qPCR) under different pathological conditions are listed in this figure.
Exosomes in Cancer
Tumor-derived exosomes may significantly influence the development and effectiveness of anti-tumor immune responses (Rupp et al., 2011). Exposure of peripherial blood mononuclear cells (PBMCs) to exosomes isolated from ascites of ovarian cancer patients impaired the cytotoxic activity of the PBMCs in the presence of dendritic cells. In addition to containing tumor antigens, exosomes secreted from cancer cells also have been shown to contain two molecules critical to immunoregulation, PD-L1 and FasL. The presence of FasL and TRAIL in exosomes may contribute to apoptosis of cells of the immune response, thereby serving as a mechanism of tumor escape from immunosurveillance (Peng et al., 2011). For example, exosomes from plasma of cancer patients have been shown to induce T cell apoptosis (Ichim et al., 2008), reminiscent of Fas-FasL counterattack that has been described in some cancers (Alderson et al., 1995; O’Connell et al., 1998). Interestingly, macrophages have been shown to influence the invasiveness of breast cancer cells through exosome-mediated delivery of oncogenic miRNAs from the macrophages to the cancerous cells (Yang et al., 2011).
What are Glypicans (HSPG’s – Heparin Sulfate Proteoglycans)?
Proteoglycans (PGs) are glycoproteins that contain long, covalently-linked unbranched chains of repeating disaccharides. HSPGs, proteoglycans with heparan sulfate (HS) glycosaminoglycan (GAG) chains, are abundant components of cells and the extracellular matrix (ECM) and as such serve diverse roles in development and pathophysiology. HSPGs act as signaling co-receptors for growth factors and morphogens, regulate the stability and distribution of signaling molecules in the ECM and chemokine gradients at sites of injury, modulate cell adhesion and motility, and affect intracellular membrane trafficking. There are three classes of HSPGs: the secreted extracellular matrix proteoglycans, the transmembrane syndecans, and the GPI-linked glypicans. In mammals, six glypicans (GPC1-6) have been identified. All have a 60-70 kDa protein core, are glycosyl phosphatidylinositol (GPI)-linked to the cell membrane, and due to their globular structure are restricted to HS-type GAG attachments.
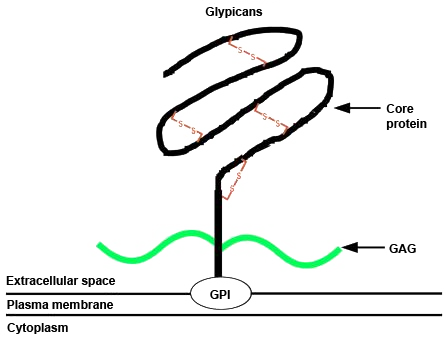
The protein is held in the plasma membrane by a GPI anchor at the carboxyl terminus. Numerous glycosoaminoglycan (GAG) attachment sites close to the membrane surface allow heparin and chondroitin sulphate chains to be attached to the core protein (shown in green). The amino terminal end of the protein is a globular structure held together by a conserved set of cysteine residues forming disulphide bridges. http://atlasgeneticsoncology.org/Genes/GC_GPC5.html

The six members of the vertebrate glypican family share a characteristic structure. Glypicans are anchored to the cell surface via a GPI linkage, have a conserved pattern of 14 cysteine residues, which contribute to intramolecular disulfide linkages, and display GAG attachment sites predominantly near the membrane. Alternative names and molecular weight of the core proteins are also indicated. http://www.rndsystems.com/Cytokine_cb08i2_GlypicansinCancer.aspx
Since glypicans act as co-receptors by facilitating the formation of ligand-receptor complexes and effectively lowering the required concentration of ligand, it is not surprising that they are expressed in the tumor environment. Glypican-1, -3 and -5 have all been associated with the tumorigenic process, mostly by affecting growth factor signaling and cell proliferation. GPC1 shows increased expression in human gliomas and glioma-derived cell lines, and acts by enhancing fibroblast growth factor (FGF) basic signaling and mitogenesis. Likewise, in pancreatic and breast cancer cells GPC1 is over-produced, and affects regulation of FGF basic and heparin-binding EGF-like growth factor (HB-EGF) signaling. Antisense depletion of GPC1 in pancreatic cancer cells also reduces their ability to form tumors in vivo. Another example of glypican-promoted proliferation of tumor cells involves GPC5. Its upregulation has a strong association with the appearance and increased cell proliferation of rhabdomyosarcoma, a malignant skeletal muscle tumor. The mechanism involves GPC5-mediated activity of FGF basic, hepatocyte growth factor (HGF), and Wnt-1.
What is Glypican-1and Its Role in Pancreatic Cancer?
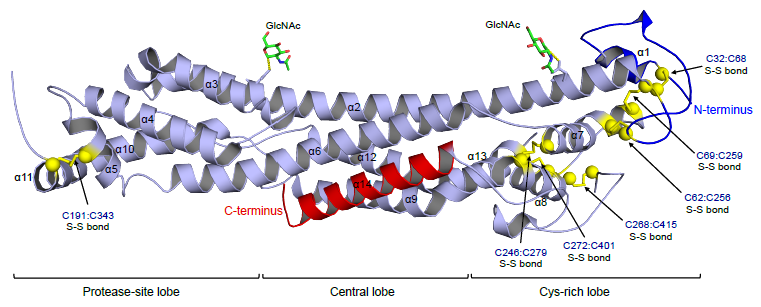
The glypican-1 gene codes for a protein of 558 amino acids with a predicted molecular weight of 62 kDa. It is a cell surface, lipid-raft-associated heparan sulfate proteoglycan (HSPG), composed of a glycosylphosphatidylinositol (GPI)-anchored core protein substituted with a three chains of heparan sulfate near its C-terminus.
Many studies have shown that GPC1 is crucial for efficient cancer cell growth, metastasis, and angiogenesis of many human and mouse cancer cell types (Ding et al., 2005; Kayed et al., 2006; Aikawa et al., 2008; Whipple et al., 2012). GPC1 is up-regulated in human cancer cells such as glioma, pancreatic and breast cancers and supports and maintains the mitogenic effect of several HS-binding growth factors (Matsuda et al., 2001; Kayed et al., 2006; Su et al., 2006). Downregulation of GPC1 results in prolonged doubling times and decreased growth of cancer cells in vitro, as well as attenuated tumor growth, angiogenesis, and metastasis in vivo.
Heparan sulfate proteoglycans (HSPGs) play diverse roles in cell recognition, growth, and adhesion. In vitro studies suggest that cell-surface HSPGs act as co-receptors for heparin-binding mitogenic growth factors. Glypican-1 is strongly expressed in human pancreatic cancer, both by the cancer cells and the adjacent fibroblasts, whereas expression of glypican-1 is low in the normal pancreas and in chronic pancreatitis. Treatment of two pancreatic cancer cell lines, which express glypican-1, with the enzyme phosphoinositide-specific phospholipase-C (PI-PLC) abrogated their mitogenic responses to two heparin-binding growth factors that are commonly overexpressed in pancreatic cancer: fibroblast growth factor 2 (FGF2) and heparin-binding EGF-like growth factor (HB-EGF). PI-PLC did not alter the response to the non-heparin-binding growth factors EGF and IGF-1. Stable expression of a form of glypican-1 engineered to possess a transmembrane domain instead of a GPI anchor conferred resistance to the inhibitory effects of PI-PLC on growth factor responsiveness. Furthermore, transfection of a glypican-1 antisense construct attenuated glypican-1 protein levels and the mitogenic response to FGF2 and HB-EGF. Glypican-1 plays an essential role in the responses of pancreatic cancer cells to certain mitogenic stimuli, that it is relatively unique in relation to other HSPGs, and that its expression by pancreatic cancer cells may be of importance in the pathobiology of this disorder.


i think you should research kevetrin trial at dana farmer. contact andrew wolanski at 617-632-6623. pancreatic cancer stabilized at 30 mm/m2g for several weeks in stage 4 patient. no dosing K at 750 mg/m2m