A drug that was rejected by the FDA in 2005 for the treatment of acute myeloid leukemia, and has also failed studies in pancreatic cancer will be further developed by a company called Kura, which in-licensed the drug from J&J.
Why?
Well, scientists are convinced that the drug could be active in a subset of patients with HRAS mutations. Prior studies did not focus on patients with this mutation, rather, all comers were enrolled. The rationale is sound as many drugs have been shown to be highly selective for cancers with certain mutations. This is currently in vogue in cancer drug development – advancing products for niches of patients defined by specific mutations.
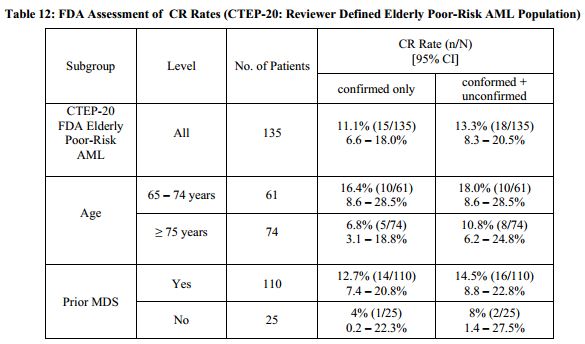
As seen in the Oncology Drug Advisory Committee (ODAC) briefing documents, the drug showed an 18% complete response rate in elderly patients with acute myeologenous leukemia AML) in Phase 2 studies. The median duration of response was 275 days. The primary toxicity is myelosuppression – 83% of patients experienced grade 3 or 4 (based on a 4-point scale) adverse events. The panel voted 7 to 4 against the product and requested additional studies.
Tipifarnib is a small molecule farnesyltransferase inhibitor. It blocks the post-translational modification of the Ras tyrosine kinase.
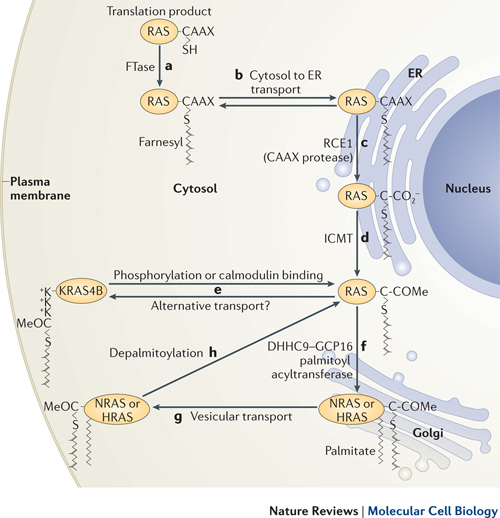
Ras post-translational modifications include the constitutive and irreversible remodelling of its carboxy-terminal CAAX motif by farnesylation, proteolysis and methylation, reversible palmitoylation, and conditional modifications, including phosphorylation, peptidyl-prolyl isomerisation, monoubiquitylation, diubiquitylation, nitrosylation, ADP ribosylation and glucosylation.
Farrnesyl is necessary to attach Ras to the cell membrane. Without attachment to the cell membrane, Ras is not able to transfer signals from membrane receptors. (See diagram.)
Kura CEO, Troy Wilson, says that advances in sequencing make it possible to pursue tipifarnib as a treatment for a subset of patients with HRAS mutations, providing a clear pathway for clinical development in a program that has plenty of cash available to complete the two Phase II studies: the first in tumors characterized by HRAS mutations, which starts in the second quarter of 2015, and a second in patients with peripheral T-cell lymphomas that launches in the third quarter of 2015. And Wilson’s team can push ahead with an ample set of safety data in hand from previous trials.
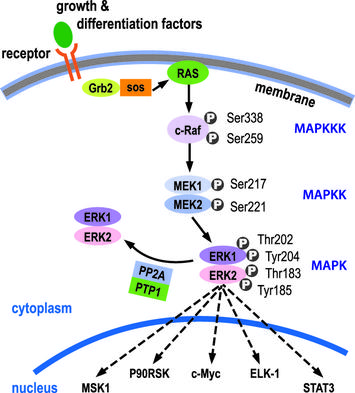
The Ras pathway is the central signal transduction pathway in the cell, and mutations in this cascade (upstream or downstream) are responsible for driving many cancers. (See – Ras signaling pathway in GeneCopoeia.)
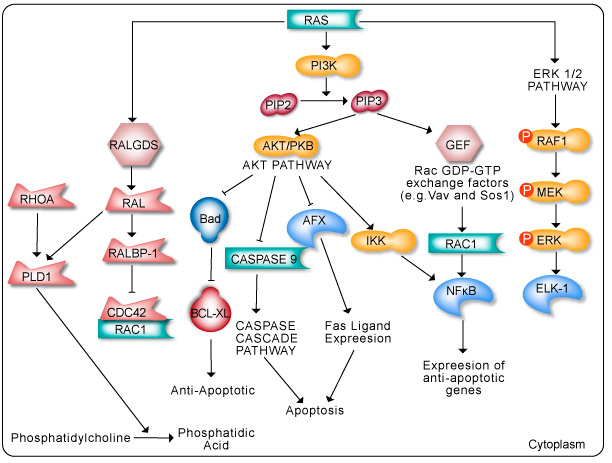
The RAS family of GTPases (HRAS, NRAS, and KRAS) comprises proteins that are highly conserved across species and has key roles in numerous basic cellular functions, including control of proliferation, differentiation, and apoptosis. Many of these Ras mediated signals are interpreted differently depending on the cell type or microenvironment receiving the stimulus. Not all of these effectors are activated in any given cell type. The first RAS effector pathway identified was the RAF-MEK-ERK pathway. This pathway is an essential, shared element of mitogenic signaling involving tyrosine kinase receptors, leading to a wide range of cellular responses, including growth, differentiation, inflammation, and apoptosis. The RAF family of proteins (Raf-1, A-Raf, and B-Raf) is serine/threonine kinases that bind to the effector region of RAS-GTP, thus inducing translocation of the protein to the plasma membrane. The second best-characterized RAS effector family is phosphoinositide 3-kinases (PI3Ks). PIP3 stimulates the AKT/PKB kinase and several of the Rac-GEF’s such as Sos1 and Vav. AKT activation inhibits apoptosis by inhibiting the actions of Bad, Caspase9 and AFX. AKT further hinders apoptosis by phosphorylating the IkB repressor of NFkB. This pathway plays important roles as mediators of RAS-mediated cell survival and proliferation.