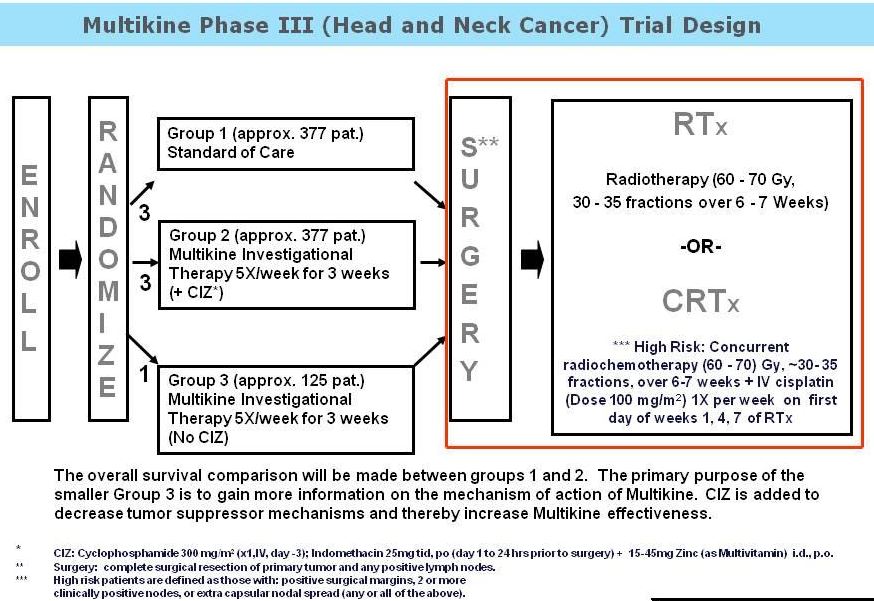
Multikine is a new product being developed by CEL-SCi for front-line use in patients with head and neck cancer. I observed a presentation at the 2015 Biotech Showcase on January 12, 2015. A late stage clinical trial is currently enrolling – Phase III Study of LI [Multikine®] Plus SOC (Surgery + Radiotherapy or Surgery + Concurrent Radiochemotherapy) in Subjects With Advanced Primary Squamous Cell Carcinoma of the Oral Cavity/Soft Palate vs. SOC Only.
It is anticipated that enrollment (880 patients) will be complete by the end of 2015. Follow-up of patients will ensue until 300 events are observed, at which time, statistical analyses will be performed. The primary endpoint of the study is overall survival; secondary endpoints include progression-free survival, loco-regional control of the tumor, and quality of life. This is the largest study ever performed in head and neck cancer, which is being supported by the US Navy.
Multikine is administered peri-tumorally, as well as peri-lymphatically at the site of the draining lymph nodes. The rationale for this is to recruit antigen presenting cells to the site of the tumor where the cancer cells are induced into the cell cycle phase to present antigens and to increase susceptibility to radiochemotherapy, and to help stimulate a robust CD8+ cell-mediated response in the lymph nodes mediated by CD4+ helper cells. Per-tumoral injection can also lead to direct cellular toxicity to the cancer, thus, Multikine is considered to be both a passive and active immunotherapy.
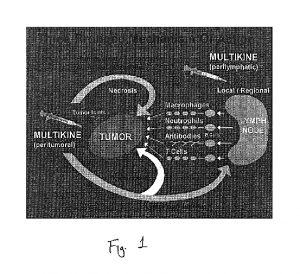
Figure 1 from US 6896879 B2 – http://www.google.com/patents/US6896879
Multikine is a complex biologic, a mixture of specific cytokines in defined relative ratios to IL-2. The cytokines were selected because they have been shown to be elevated in patients who have experienced response to therapy (of interventions other than Multikine, such as, radiotherapy, chemotherapy, and surgery). The premise is that administration of the same collection of cytokines could induce more patients to respond to treatment with surgery and radiochemotherapy.
Clinical and pathology data from Phase I and Phase II clinical trials with the Multikine investigational therapy published in peer reviewed scientific journals (Timar et al 2003 and 2005) suggest that Multikine has the potential to produce an anti-tumor response, as the reported data from these early-stage studies suggests:
- Multikine potentially may recognize and/or bind to multiple (different) antigens (or receptors) on the cancer cells.
- Multikine potentially may directly affect/kill cancer cells: The various cytokines present in the Multikine investigational therapy, such as TNF, IL-1, along with other cytokines, could be responsible for this potential activity.
- Multikine may signal the immune system to produce an anti-tumor immune response: Clinical data (reported from Phase II studies) also suggest that Multikine could change the type of cells that infiltrate and attack the tumor from CD-8 cells to predominantly CD-4 cells. These CD-4 cells have the potential to bring about an anti-tumor immune response (Timar et al 2005).
- Although the clinical significance of such a response if confirmed is not yet established, this could be important because the tumor is able to shut down the infiltrating CD-8 cells, but preliminary evidence seems to suggest a potential inability to shut down the CD-4 cell. In addition, preliminary data suggests that CD-4 cells appear to help break “tumor tolerance,” thereby potentially allowing the immune system to recognize and address the tumor. The normal immune system is made ‘blind’ to tumor cells, in part, because the tumor cells are derived from the body’s own cells, and thus the body ‘thinks’ of the tumor as ‘self’, a phenomenon also known as ‘tumor tolerance’.
- Multikine may have the potential to render the remaining cancer cells susceptible to radiation and chemotherapy treatment (Timar et al 2003).
The different cytokine and small biological molecules that constitute Multikine are derived from the lectin (PHA) in vitro stimulation of human peripheral blood mononuclear cells that include T cells, B cells, and macrophages.
Mulitkine contains the following cytokines: TNF-α (and β), IFN-g, IL-1 (α and β), IL-2, IL-3, IL-6, IL-8, IL_16, MIP-1 (α and β), GM-CSF, EGF, PGE2, TxB2, and RANTES.
Multikine does not contain the following cytokines and other small biologically active molecules: IL-4, IL-7, and IL-15, TfR, sICAM, PDGF-AB, IFN-α, EPO, LTC 4, TGF-β2, FGF basic, Angiogenin, sE-selectin, SCF, and LIF. Multikine® contains only trace quantities (just above the level of detection of the assay) of IL-12, and LTB 4.
I hope the Phase III trial is positive.