Last week, Amgen (Onyx) announced negative news from its FOCUS clinical trial of multiple myeloma drug Kyprolis. Let’s put this into perspective.
On July 20, 2012, Kyprolis (carfilzomib) won accelerated approved to treat patients with multiple myeloma who had failed prior treatment with Velcade (bortezomib) and thalidomide. Kyprolis and Velcade have a similar mechanism of action – they inhibit the proteasome, a cellular system that degrades unwanted proteins.
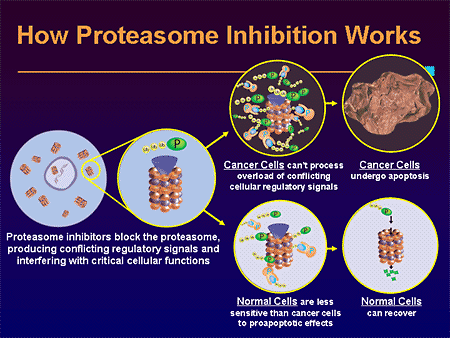
In multiple myeloma, B cells are producing large amounts of antibody, rapidly. Frequently, the antibodies become miss-folded and require destruction by the cell. If proteasome degradation is blocked, destruction of large macromolecules cannot take place; this back-up causes the cells to enter into apoptosis. (See The Proteasome, Presented by William S. Dalton, PhD.)
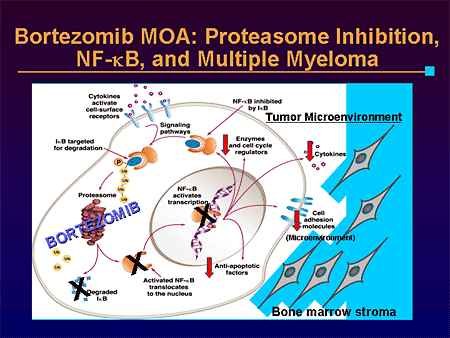
When proteasome is inhibited, another molecule IkB (Inhibitor of NFkB) cannot be destroyed after being phosphorylated by IKK. IkB then stays in the cytoplasm and inhibits NFkB. Cancer cells are particularly dependent on NFkB, which induces cyclin-D1, myc, BCL-2, and inhibitors of apoptosis (IAP).
As if that weren’t enough to induce cancer cells, peculiar to multiple myeloma cells, NFk-B also:
- Increases production of IL-4 and IL-6, which are autocrine drivers of growth
- Induces VEGF
- Induces adhesion molecule expression required for heterotypic interactions with bone marrow stem cells
Accelerated approval allows for the approval of a cancer drug based on a “surrogate endpoint. In the case of Kyprolis, that was an overall response rate 22.9% with a median duration of 7.8 months in a non-randomized Phase 2b trial. The FDA website states that when accelerated approval is granted, the drug company will still need to conduct studies to confirm that tumor shrinkage actually predicts that patients will live longer. These studies are known as confirmatory trials, and must be randomized to another treatment in order to determine the impact on survival.
The Phase 3 FOCUS study of Kyprolis reported last week was a randomized study conducted in 315 patients who had failed prior therapy (median of 5 prior regimens), but were randomized to either Kyprolis or cyclophosphamide (alkylating agent) and dexamethasone (anti-inflammatory steroid). The study did not meet its endpoint of improving overall survival.
To further confuse matters, the interim results of another phase 4 randomized trial are positive. The ASPIRE Phase 3 clinical trial (CArfilzomib, Lenalidomide, and DexamethaSone versus Lenalidomide and Dexamethasone for the treatment of Patients with Relapsed Multiple Myeloma) met its primary endpoint of progression-free survival (PFS). Patients treated with Kyprolis for Injection in combination with Revlimid (lenalidomide – similar to thalidomide) and low-dose dexamethasone lived significantly longer without their disease worsening (median 26.3 months) compared to patients treated with Revlimid and low-dose dexamethasone (median 17.6 months) (HR=0.690, 95 percent CI, 0.570, 0.834, p<0.0001).
Which is better, Kyprolis or Velcade? It is an interesting question because they both inhibit the 20S proteasome core, but Kyprolis does so irreversibly, and Velcade reversibly. A head to head Phase 3 ENDEAVOR study is underway – ENDEAVOR (RandomizEd, OpeNLabel, Phase 3 Study of Carfilzomib Plus DExamethAsone Vs Bortezomib Plus DexamethasOne in Patients With Relapsed Multiple Myeloma) trial is an 888 patient study evaluating Kyprolis in combination with dexamethasone, versus Velcade (bortezomib) with dexamethasone in patients whose multiple myeloma has relapsed after at least one, but not more than three prior therapeutic regimens.
The primary endpoint of the ENDEAVOR trial is progression-free survival. Secondary endpoints include overall survival, overall response rate, duration of response, and safety. Patients will be randomized to receive Kyprolis intravenously (20mg/m2 on days 1 and 2 of cycle 1 only, then 56 mg/m2 subsequently) with low-dose dexamethasone (20mg), versus bortezomib (1.3 mg/m2) with low-dose dexamethasone. Bortezomib can be administered subcutaneously or intravenously at the discretion of the investigator and in accordance with regulatory approval of bortezomib.
In fact, there are 89 clinical trials of Kyprolis – see clincaltrials.gov.