IDO ( indoleamine 2,3-dioxygenase) is a molecule that oxidizes tryptophan, which is needed by cytotoxic T-cells. When cancer cells and T-reg cells secrete IDO in the vicinity of cytotoxic T cells, antitumor activity is abrogated.
Several companies (INCYTE , BMS,) are developing IDO inhibitors, which function in a similar fashion to immune checkpoint inhibitors. Dendritic cells also express IDO, and when this is done in the context of antigen presentation, induction of T-reg cells occurs – see Nature Reviews Immunology 4, 762-774 (October 2004)
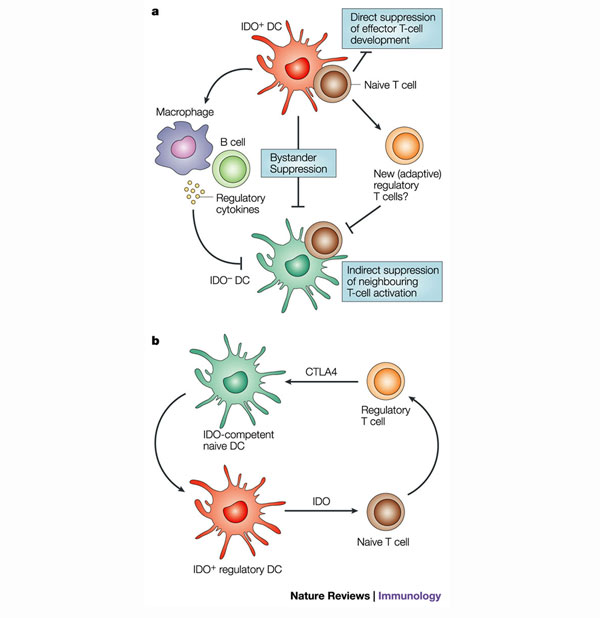
a | Potential mechanisms for the ‘bystander’ effects of indoleamine 2,3-dioxygenase (IDO)-expressing cells. In vitro and in vivo studies indicate that even small numbers of IDO+ dendritic cells (DCs) can have widespread suppressive effects on T cells that recognize antigen presented by IDO- DCs. The figure depicts hypothetical mechanisms by which suppression of adjacent T cells might occur. There could be bystander suppression mediated by the IDO-expressing DC itself — for example, through toxic metabolites, widespread local tryptophan depletion or IDO-induced regulatory cytokines. Neighbouring cells (for example, macrophages, regulatory T or B cells) might similarly be induced by IDO-responsive signalling pathways to secrete regulatory cytokines. Naive T cells (CD4+ or CD8+) might be biased by IDO+ DCs to adopt a regulatory phenotype. Any of these effects could then suppress nearby T cells responding to IDO- antigen-presenting cells (APCs). The effect of IDO would therefore be to convert the local tissue microenvironment into a tolerizing milieu, even for antigens presented by other, normally immunogenic APCs. b | Model of a self-amplifying regulatory network involving interactions between IDO-competent DCs and regulatory T cells. The generation of regulatory T cells by IDO-expressing DCs could be a potent mechanism, as these regulatory T cells might in turn create other tolerogenic DCs through the induction of IDO expression by cytotoxic T lymphocyte antigen 4 (CTLA4) interactions. This could provide one possible molecular mechanism for epitope spreading and ‘infectious’ tolerance, as described in other systems.
Regen BioPharma is developing an autologous dendritic cell vaccine to silence IDO expression by dendritic cells. called dCellVax (US patent # 8,389,708). The IDO gene is silenced using an siRNA and then re-infused. The company is preparing an FDA IND (Invesitgational New Drug Application) to begin clinical studies. siRNA’s work by associating with the Dicer enzyme and RISC complex to then bind to complementary mRNA (IDO) and degrade it. This can be done with liposomal preparations of siRNA molecules (transient suppression) or via plasmid transfection (prolonged suppression) – see Gene Silencers – RNA Interference.
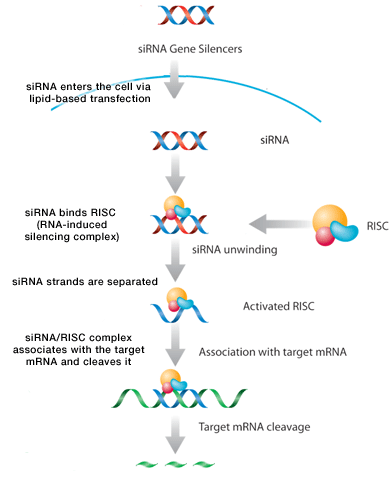
RNA interference (RNAi) was first identified in C. elegans by Nobel laureates Fire and Mello (1), and now represents one of the most promising discoveries in molecular biology. Endogenous RNAi activity has been linked to the regulation of transposon mobility (2), the determination of gene expression profiles (3) and cell fate (4), and is a crucial component of the innate cellular defense against viral infection in vivo(5). Three unique RNAi mechanisms controlling target gene expression have been demonstrated. RNAi regulates gene transcription by modifying heterochromatin formation (6). RNAi exercises two forms of post-transcriptional control. First RNAi can inhibit the translation of target mRNA (7) and second, RNAi can direct target mRNA destruction through the RISC complex (8). DICER first processes dsRNA leaving a two nucleotide long 3′ overhang. This primes the dsRNA for binding to the RISC complex and leads to the activation of the enzyme activity of argonaute, the RNAse component of the RISC complex that destroys one of the RNA strands. The remaining guide strand, through complementary binding, then leads the RISC complex to associate with and cleave target RNA molecules.

