March begins National Kidney Month. The IHS Library can provide you with many resources regarding this very important and vital organ. Check out some of our resources, including organizational links, eBooks, journals and articles, with regards National Kidney Month.
General Kidney Information:
To know more about kidney disease topics, check the following:
- National Institute of Diabetes and Digestive and Kidney Diseases
https://www.niddk.nih.gov/health-information/kidney-disease
- National Kidney Month Toolkit via the National Institute Diabetes and Digestive and Kidney Diseases
https://www.niddk.nih.gov/health-information/community-health-outreach/national-kidney-month/toolkit
- MedlinePlus.gov: Kidney Diseases
https://medlineplus.gov/kidneydiseases.html
Chronic Kidney Disease by the numbers (CDC):
- Kidney diseases are a leading cause of death in the United States.
- About 37 million US adults are estimated to have CKD, and most are undiagnosed.
- 40% of people with severely reduced kidney function (not on dialysis) are not aware of having CKD.
- Every 24 hours, 360 people begin dialysis treatment for kidney failure.
- In the United States, diabetes and high blood pressure are the leading causes of kidney failure, accounting for 3 out of 4 new cases.
- In 2019, treating Medicare beneficiaries with CKD cost $87.2 billion, and treating people with ESRD cost an additional $37.3 billion.
https://www.cdc.gov/kidneydisease/basics.html
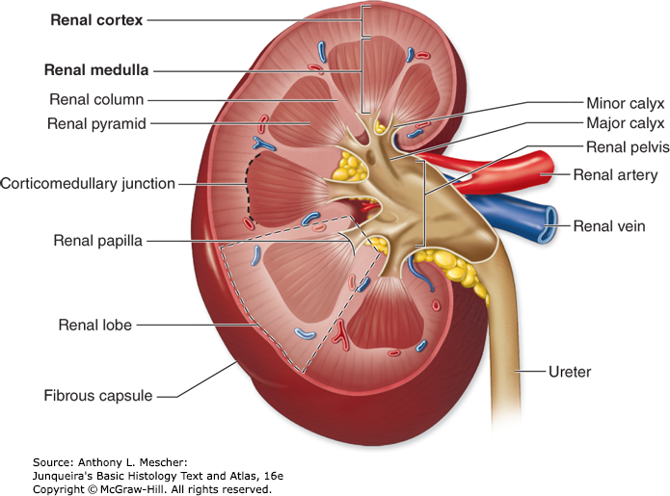
Anatomy Resources
- Acland’s Video Atlas of Human Anatomy
https://aclandanatomy.com/multimediaplayer.aspx?multimediaid=10528651
- Anatomy TV powered by Primal Pictures (Kidney 3D View)
- AccessMedicine Human Anatomy Modules
- Anatomy Books
https://library.shu.edu/Phase1/anatomy
Books
The IHS library has a large of collection of eBooks pertaining to the kidney. Below are just some examples of eBooks that are available to view:
AccessMedicine Collection of McGraw Hill books on Nephrology
Additional eBooks
Methods in kidney cell biology: Part A
Methods in kidney cell biology: Part B
National Kidney Foundation’s primer on kidney diseases

Heptinstall’s pathology of the kidney

Clinical Overviews and Evidence-Based Information
- Critically Appraised Topical Information and Clinical Overviews can be found here:
Databases
Guidelines
NKF KDOQI clinical practice guidelines
National Institute for Health and Care Excellence
Images (multimedia)
IHS library resources regarding images and kidney’s
Infographics
Journals
Check here to see the following journals that are present in the IHS library holdings through BrowZine. The IHS library subscribes to some core Nephrology journals including the American Journal of Nephrology and American Journal of Kidney
Nephrology Journals Via BrowZine
Contact your IHS Librarian!
For more information regarding library resources and services reach out to your IHS librarian to set up a consultation or a quick chat!



 September 18 – 24 is
September 18 – 24 is  Fun Home
Fun Home  Alice’s Adventures in Wonderland
Alice’s Adventures in Wonderland 
 Me and Earl and the Dying Girl: a Novel
Me and Earl and the Dying Girl: a Novel Between the World and Me: Notes on the First 150 Years in America
Between the World and Me: Notes on the First 150 Years in America Pedagogy of the Oppressed
Pedagogy of the Oppressed The Call of the Wild
The Call of the Wild The Great Gatsby
The Great Gatsby White Fragility: Why It’s So Hard for White People to Talk About Racism
White Fragility: Why It’s So Hard for White People to Talk About Racism The immortal Life of Henrietta Lacks
The immortal Life of Henrietta Lacks The Autobiography of Malcolm X
The Autobiography of Malcolm X


 Kyle Downey was awarded the “Opportunity Meets Innovation (OMI) Challenge Grant” through Seton Hall University along with co-researchers, Dr. Lauren Snowdon and Dr. Angela Lis of the Physical Therapy program. This grant was designed to create interdisciplinary and collaborative research opportunities among faculty and students from different academic disciplines.
Kyle Downey was awarded the “Opportunity Meets Innovation (OMI) Challenge Grant” through Seton Hall University along with co-researchers, Dr. Lauren Snowdon and Dr. Angela Lis of the Physical Therapy program. This grant was designed to create interdisciplinary and collaborative research opportunities among faculty and students from different academic disciplines. Peggy Dreker was awarded “University Libraries Faculty Researcher of the Year” through Seton Hall University. Peggy received this honor at a March 31st Faculty Researcher and Teacher of the Year Awards luncheon presented by the Office of the Provost.
Peggy Dreker was awarded “University Libraries Faculty Researcher of the Year” through Seton Hall University. Peggy received this honor at a March 31st Faculty Researcher and Teacher of the Year Awards luncheon presented by the Office of the Provost.