We’re pleased to announce another enhancement to the user experience for IHS students, faculty, and staff: Libkey Link for PubMed. To quote Third Iron, the vendor we’ve partnered with for this feature, Libkey Link “brings one-click access to PDF articles in PubMed, dramatically simplifying workflow and improving user experience. No more confusion over what full text source to pick, no more waiting for different pages to load hunting for the PDF button.” We’ve implemented LibKey Link to cut out a couple of steps in the old process, making it easier and faster for you to get the article PDF you’re looking for. As always, if you need any help with this feature (or anything else information-related), contact your IHS librarian.
Month: April 2019
Improvements to the SHUhealth search tool
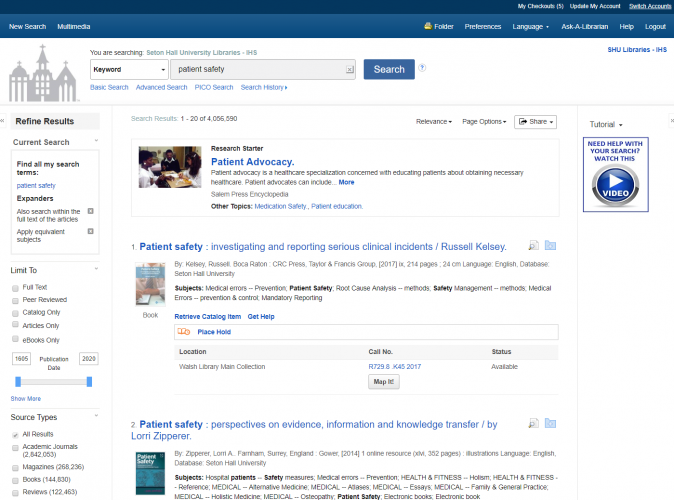
SHU Health is a tool that allows IHS Library users to search both the catalog (books, videos, CDs, etc.) and articles simultaneously. We are excited to announce a number of improvements to the interface to streamline the experience for you.
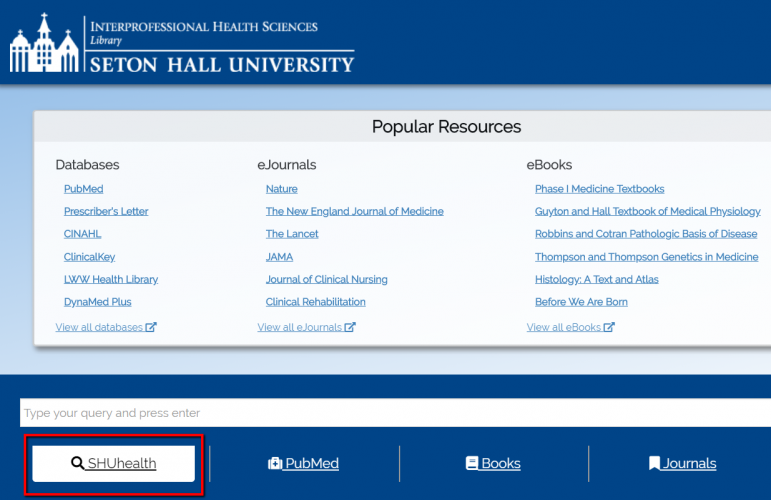
The updated interface is significantly simpler, reducing clutter by removing little-used and unnecessary features. IHS librarians reached out to our partner EBSCO, which manages the interface, to suggest the changes.
We’re interested in continually improving your experience with IHS Library website and other library interfaces. If you have an improvement to suggest or notice a bug we need to fix, email me at andrew.hickner AT shu.edu.
Cardiac Exosomes to the Rescue
In an extremely exciting study that appeared last month in the journal Nature Communications[1], scientists from the University of Alabama at Birmingham, and Huazhong University of Science and Technology in Wuhan, China, showed that after myocardial infarction (~heart attack), heart tissue release tiny membrane-bound vesicles called exosomes, specifically loaded with genetic material designed to promote cardiac tissue repair. Such restoration is mediated by bone marrow progenitor cells that have been released from their sequestered bone-specific existence by the information carried in the newly arrived exosomes.
Several hundred thousand Americans suffer heart attacks each year, making it critical for the medical and scientific communities to understand this potentially transformative study. To begin our analysis, let’s begin with exosomes – tiny membrane-enclosed vesicles released from many cell types. Thought to mediate communication/cell-cell signaling activities, exosomes leave cells loaded with proteins, lipids, DNA, mRNA, and microRNAs among other potentially bioactive molecules. Exosomes are synthesized within the endosome-lysosome system of cells – emerging cargo loaded and poised to deliver their contents locally, or at a distance. It is perhaps not surprising that several pharmaceutical companies – recognizing the potential drug delivery platform that exosomes represent, are pursuing the tiny vesicles in several biomedical contexts.
Once released, how the nascent exosomes hone to specific target tissues and cells is unclear. Perhaps the proteins assembled into their membranes – distinct to each type of exosome – play a role in this discrimination. Also not well understood is the nature of the exosome-target cell interaction. Is it a receptor-ligand-like binding event that triggers signaling events and results in fusion and integration of exosomal contents? Or is it a more basic fusion of two membranes held in close apposition? What about a straightforward endocytic process whereby the exosome is taken up and released within the newly formed endosome?
Back to the study at hand – the investigative team identified the exosomes released from damaged heart muscle as containing specific microRNAs (called myocardial microRNAs or myo-miRs). Recall microRNAs are short, non-coding RNAs with the capacity to (negatively) regulate gene expression. The myo-miRs under consideration here do just that – they very effectively block activity of the chemokine receptor, CXCR4 in bone marrow progenitor cells. Such inhibition allows such stem-like cells to escape their seclusion, enter the bloodstream, and travel to the injured heart…“exosomes to the rescue.” There, repair processes are initiated.
Knowing this cell biology, there must be ways to better harness exosomes to improve cardiac repair. Investigators are sure to pursue this quickly. Almost as assuredly, scientists will also be looking for other examples of exosomes and their bioactive contents as biomarkers for pathology, and as potential rescue mechanisms for disease’s damaging wrath.
SRT – April 2019
[1]Cheng M, et al., Nat Commun. (2019) doi: 10.1038/s41467-019-08895-
