Indoximod + Keytruda looks promising in Phase 2 advanced melanoma
IDO (indoleamine-2,3-dioxygenase) is an intracellular enzyme found in antigen presenting cells that mediates immune suppression in the tumor microenvironment.
Indoleamine-2,3-dioxygenase (IDO) is an intracellular heme-containing enzyme that initiates the first and rate-limiting step of tryptophan degradation along the kynurenine pathway. In mammalian organisms, tryptophan is an essential amino acid for cell survival; it cannot be synthesized de novo.
An important mechanism by which IDO affects T-cell activity is that the consumption of local tryptophan inhibits mechanistic target of rapamycin (serine/threonine kinase) complex 1 (mTORC1) as well as the T-cell receptor regulatory kinase, protein kinase C theta (PKC-θ), both of which are regulatory targets of the master amino acid-sensing kinase, glucokinase (GLK1). mTORC1 inhibition can trigger a response that includes activating autophagy, leading to anergy in T cells in the tumor microenvironment.
IDO expression can further induce a stress response in cells via activation of general control nondepressible-2 (GCN2). Tryptophan degradation by IDO causes local tryptophan deficiency, leading to the accumulation of uncharged tryptophan transfer ribonucleic acid (tRNA) in cells. GCN2, a stress-response kinase, is stimulated by elevations in uncharged tRNA, and its activation can limit or alter protein translation and prevent T-cell activation. Furthermore, activation of GCN2 promotes de novo Treg differentiation and enhances Treg activity, resulting in profound immunosuppressive microenvironment.
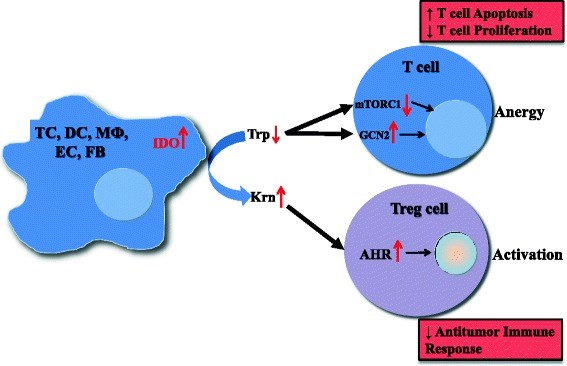
Figure 1. Mechanisms of IDO pathway activity in immune tolerance. The enzyme IDO catalyzes the initial and rate-limiting step in the catabolism of tryptophan along the kynurenine pathway. Accordingly, IDO can provoke tryptophan shortage, which results in mTORC1 inhibition and GCN2 activation, in turn leading to anergy of effector T cells. Tryptophan’s degradation leads to production of bioactive kynurenine pathway compounds, which activate the AHR, resulting in promotion of Treg differentiation. TC, tumor cell; DC, dendritic cell; MФ, macrophage; EC, endothelial cell; FB, fibroblast; IDO, indoleamine-2,3-dioxygenase 1; Trp, tryptophan; Krn, kynurenine; mTORC1, mechanistic target of rapamycin (serine/threonine kinase) complex 1; GCN2, general control nondepressible-2; AHR, aryl hydrocarbon receptor; Treg, regulatory T cell. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678703/
Rationale for IDO inhibition plus checkpoint inhibition
Although patients who respond to treatment with checkpoint inhibitors have excellent outcomes, most patients do not respond. The goal, then, is to add Indoximod with the hope of squelching Treg cells in the microenvironment so that immune checkpoint blockade can induce responses and excellent outcomes in more patients.
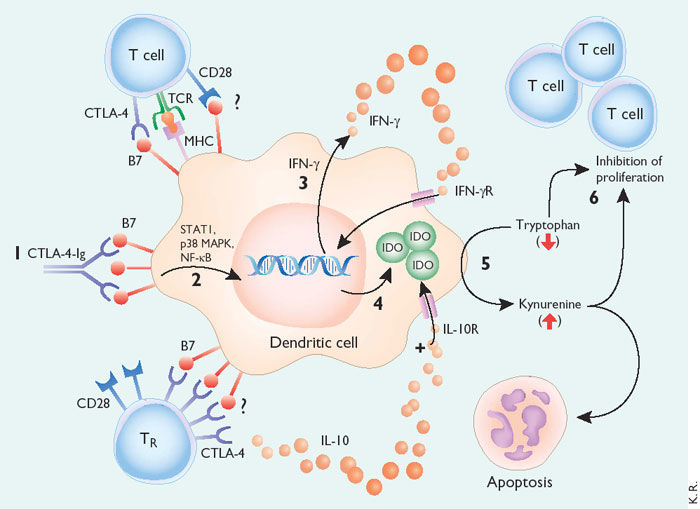
Figure 2. Model of tolerance induction by B7-dependent signaling in DCs.
CTLA-4−Ig binding to DCs induces DC signaling via B7 (1). In a signal transduction pathway that is dependent on STAT1, p38 MAPK and NF- B (2), the DC is stimulated to produce IFN- (3). IFN- acts in an autocrine or paracrine manner to promote up-regulation of IDO (4). IDO catalyzes the degradation of tryptophan to kynureine and subsequent catabolic byproducts (5). Local decreases in availability of tryptophan and the presence of its catabolic byproducts inhibit T cell proliferation and may induce apoptotic death (6). Inhibition of clonal expansion leads to a functional state of tolerance. This pathway may be accentuated by the presence of IL-10, which sustains IDO expression in DCs. The role played by cell surface CTLA-4 or CD28 in inducing signaling and IDO activity is not known, but it may mimic the activity of CTLA-4−Ig. TR cells secrete IL-10 and may induce these B7-dependent DC signaling pathways. http://www.nature.com/ni/journal/v3/n11/fig_tab/ni1102-1056_F1.html?foxtrotcallback=true
IDO inhibition + checkpoint blockade in advanced melanoma
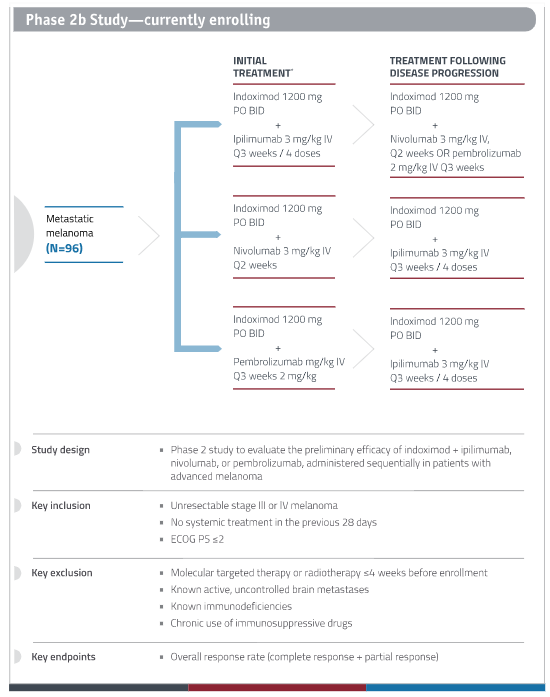
New Link Genetics is conducting a phase 2b clinical study of Indoximod in patients with advanced melanoma who are receiving CTLA-4 and PD-1 checkpoint inhibitors – Yervoy (ipilimumab; CTLA-4), Opdivo (nivolumab; PD-1), or Keytruda (pembrolizumab; PD-1), all of which are approved for advanced melanoma by the FDA. Important inclusion/exclusion criteria include:
- Inclusion Criteria –
- Unresectable Stage III or Stage IV melanoma.
- Patients must have measurable disease, defined as lesions that can be accurately measure in in 2 perpendicular diameters with at least one diameter > 20mm and the other >10mm on conventional CT or MRI or 10mm x 10 mm by spiral CT.
- No systemic treatment in the previous 28 days.
- Exclusion Criteria –
- Patients who have had prior therapy with immune checkpoint inhibition or or indoximod are excluded from the trial. Pre-treatment with other immune modulators is allowed in the phase 1 component of the study only. For the Phase II component, patients are excluded if they have had prior therapy with or immune-stimulating agents including interleukin-2, interferons, CTLA-4 or PD1 antagonists, CD40 or CD137 agonist, or cancer therapeutic vaccines in any prior line for metastatic disease. Interferons used in the adjuvant setting are allowed (Phase 1 or 2 component).
Results in 51 patients treated with Indoximod + Keytruda demonstrate an overall response rate of 61% and median progression-free survival of 12.9 months.

Table 1. Indoximod + Keytruda – Third International Cancer Immunotherapy Conference. http://investors.linkp.com/releasedetail.cfm?ReleaseID=1039462
These results compare quite favorably to Keytruda, alone, based on the KEYNOTE-006 registration trial in which overall response rate was 34% and median progression-free survival was 5.1 months.
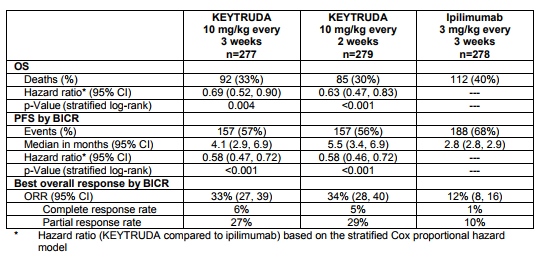
Table 2. Ketruda in advanced melanoma – KEYNOTE-006. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
Phase 3 study of Indoximod + Keytruda or Opdivo
New Link is planning a Phase 3 trial that will include 600 patients with
Stage III unresectable and metastatic stage IV melanoma. The trial will have a one to one randomization between indoximod plus KEYTRUDA (pembrolizumab) or OPDIVO (nivolumab) compared to single agent PD-1 inhibitor. The co-primary endpoints of the study are PFS by RECIST criteria and Overall Survival (OS).