Two companies – Biontech, RNA vaccine and Neon Therapeutics, peptide vaccine – reported very positive results of neo-antigen vaccines in patients with refractory metastatic melanoma.
What are neo-antigens and why are they important?
Tumors that have become invasive, metastatic, and refractory have not only accumulated a sufficient number of mutations to express the cancer phenotype, they also have developed a highly mutable genome. Their ability to survive in the face of treatment is driven by their ability to mutate. Neo-antigens are mutated forms of proteins made by the cell. Whereas an immune response may have been triggered against tumors over-expressing wild-type proteins, re-expressing embryologic proteins, or expressing mutant proteins that help to initially confer the cancer phenotype, the immune system becomes functionally tolerized to them through the emergence of Treg cells and other mechanisms. Neo-antigens are important because the immune system, ostensibly, has yet to mount an attack against these specific sequences, therefore, the body has not yet to be tolerized to them. Neo-antigens represent an excellent opportunity to exploit.
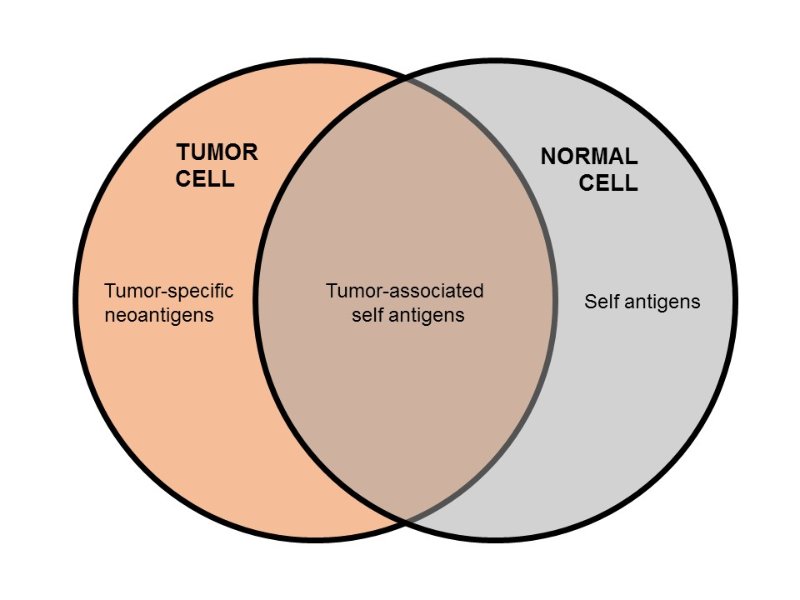
Figure 1. Tumor neoantigens (or tumor-specific antigens) represent a small fraction of antigens that arise from mutations which only occur in tumor cells. They are ideal targets for cancer immunotherapy, because neoantigens are not found in normal cells, and targeting them reduces the risk of unwanted adverse effects (see figure below). Also, neoantigens (unlike self antigens) should not be tolerized, since they are unique to tumor cells. https://www.linkedin.com/pulse/race-discover-neoantigens-cancer-immunotherapy-peter-ho
It has been shown that patients whose tumors express a large number of non-synonymous mutations develop more robust immune responses and have longer progression-free intervals to immune checkpoint inhibition therapy:
Tumor neoantigens are the consequences of the genetic alterations accumulated by cancer cells during the tumorigenesis process. They have been recently demonstrated to arise from various processes that alter the open reading frame (ORF) sequences in the genome. Not only missense mutations but also fusion transcripts, frameshifts, and stop losses can also potentially create altered ORFs (i.e., neoORFS) encoding novel stretches of amino acids that are not present in the normal genome. With the increasing accessibility to next-generation sequencing technologies combined with bioinformatics improvements, the process of neoantigen discovery has accelerated. The use of whole-exome sequencing combined with in silico peptide translation has become a promising approach to detect potential patient-specific neoantigens. Moreover, immunogenicity of the discovered neoantigens can be assessed using peptide immunogenicity prediction algorithms and high-throughput assay strategies.
Neoantigens are now increasingly recognized as immunodeterminants, as there is strong evidence that they participate in early tumor recognition and destruction by antigen-specific T cells in the context of immunotherapy treatment. In recent studies, two teams independently reported that tumors accumulating a high number of somatic nonsynonymous mutations were more likely to have durable benefit from antibody-based checkpoint blockade immunotherapy. This observation is consistent with the hypothesis that recognition of neoantigens, formed as a consequence of somatic mutations, by the host immune cells are important for the activity of such therapy. This is of particular interest as data from The Cancer Genome Atlas (TCGA) and others suggest that tumors with high mutational burden are associated with cytotoxic T-cell markers, such as CD8A expression.
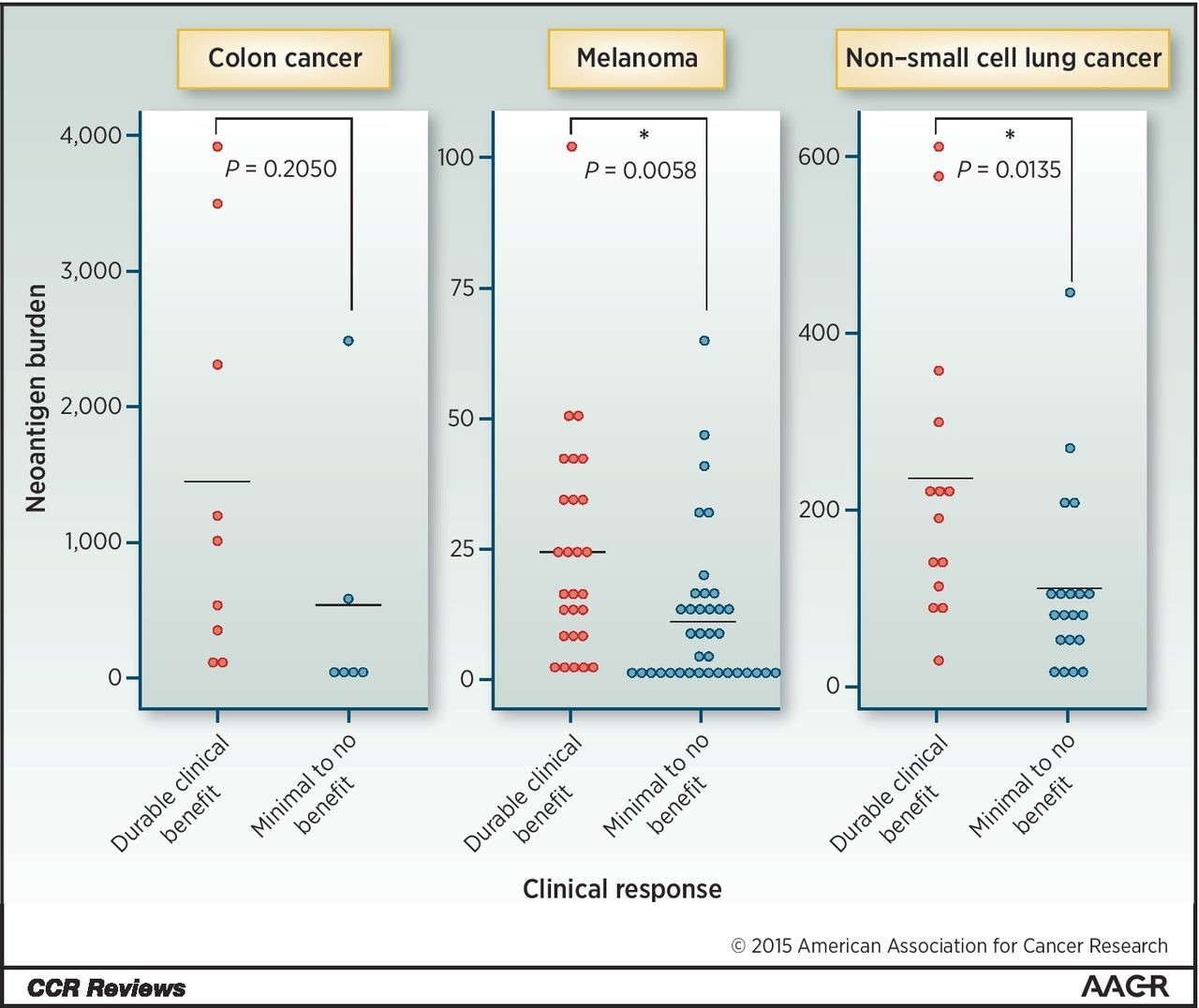
Figure 2. Neoantigen burden and tumor response. Neoantigen load according to clinical benefit to checkpoint blockade immunotherapy. Data from Le et al., Snyder et al., and Rizvi et al. are shown. Neoantigen prediction algorithms vary in the studies. http://clincancerres.aacrjournals.org/content/22/4/807
Tumors harboring potentially deleterious mutations in the mismatch repair pathway (MMR), base excision repair pathway (BER), or nucleotide excision repair pathway (NER) were found to carry a high number of candidate neoantigens and associated with clinical benefit from immune checkpoint inhibitor therapy. High mutation burden is present in other types of cancer such as uterine, bladder, head and neck cancers, and stomach cancer and could portend successful responses to checkpoint blockade immunotherapy. Furthermore, the potential of neoantigens in cancer immunization holds promise as a novel therapeutic modality.
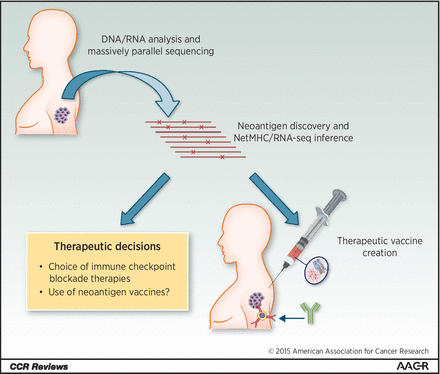
Figure 3. Neoantigen identification and vaccination. A paradigm of neoantigen utility. Proposed strategy to use neoantigen repertoire in a predictive and therapeutic manner. Sequencing of tumor DNA leads to the discovery and assessment of the “neoantigenome.” In parallel, creation of a personalized therapeutic vaccination could also be possible. http://clincancerres.aacrjournals.org/content/22/4/807
Biontech’s approach
Collaborating with Genentech, Biontech rapidly sequences tumor specimens to identify the “mutanome,” the unique mutations present in sample. Ten sequences that are unique to the tumor and predicted to evoke a strong T-cell response are selected and synthesized into an mRNA vaccine.
In a recent paper, five patients with metastatic melanoma received the mRNA vaccine – CD8+ and CD4+ responses were precipitated:
All patients developed T cell responses against multiple vaccine neo-epitopes at up to high single-digit percentages. Vaccine-induced T cell infiltration and neo-epitope-specific killing of autologous tumour cells were shown in post-vaccination resected metastases from two patients. The cumulative rate of metastatic events was highly significantly reduced after the start of vaccination, resulting in a sustained progression-free survival. Two of the five patients with metastatic disease experienced vaccine-related objective responses. One of these patients had a late relapse owing to outgrowth of β2-microglobulin-deficient melanoma cells as an acquired resistance mechanism. A third patient developed a complete response to vaccination in combination with PD-1 blockade therapy. Our study demonstrates that individual mutations can be exploited, thereby opening a path to personalized immunotherapy for patients with cancer.
Neon Therapeutics’ Approach
Neon is working with the Broad Institute to identify epitopes (peptide vaccines) comprised of 20 neoantigens that have high affinity binding to HLA molecules. In a recent study in six patients with metastatic melanoma:
Vaccine-induced polyfunctional CD4+ and CD8+ T cells targeted 58 (60%) and 15 (16%) of the 97 unique neoantigens used across patients, respectively. These T cells discriminated mutated from wild-type antigens, and in some cases directly recognized autologous tumour. Of six vaccinated patients, four had no recurrence at 25 months after vaccination, while two with recurrent disease were subsequently treated with anti-PD-1 (anti-programmed cell death-1) therapy and experienced complete tumour regression, with expansion of the repertoire of neoantigen-specific T cells.
Neon is planning additional studies in combination with BMS’ Opdivo (nivolumab). The rationale is to provoke a first wave immune response against neo-antigens using the peptide vaccine and to block its abrogation with the PD-1 inhibitor in order to maintain a prolonged robust immune response against the tumor. The response to PD-1 inhibition following relapse after peptide vaccination strongly supports the rationale of combining the two approaches for maximal benefit.

This is great so proud of my school