Cancer and autoimmune disorders are opposite sides of the same coin – cancer is a result of hypo-active immunity, whereas, autoimmune diseases is the result of hyper-active immunity. This is dramatically illustrated in examining side effects in patients with melanoma who receive checkpoint therapy with ipilimumab (Yervoy), which acts in the early stages of T-cell activation and priming, and nivolumab (Opdivo), which acts in the later stages of T-cell activation in the tumor microenvironment.
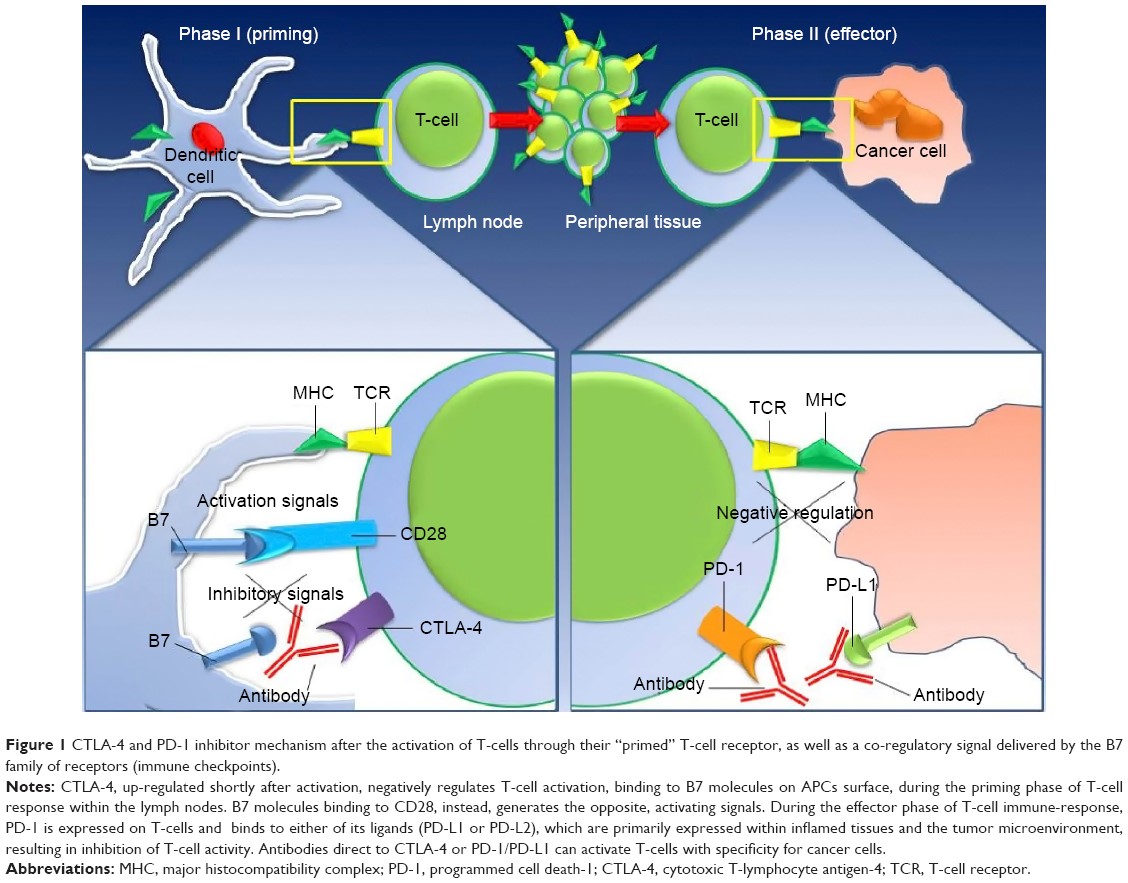
Figure 1. https://www.dovepress.com/new-developments-in-the-management-of-advanced-melanoma-ndash-role-of–peer-reviewed-fulltext-article-OTT
Checkpoint inhibitors block the abrogating signals that attenuate immune responses that allow cancers to thrive, grow, and metastasize. Blocking the blockers, so to speak, augment immune responses against the tumors. However, checkpoint inhibitors are not selective to cytotoxic T-cells directed at cancers. Rather, they act on all T-cells raising the possibility of unwanted immune responses against normal tissues, that is autoimmune disease.
In a study of 576 patients with advanced melanoma that received nivolumab, 71% of patients experienced treatment related adverse events, the most common of which were fatigue (25%), pruritus (17%), diarrhea (13%), and rash (13%).
About half of the patients experienced select adverse events — most frequently skin-related, gastrointestinal, endocrine, and hepatic, and typically mild to moderate in intensity. Grade 3 to 4 select adverse events occurred in 4% of patients. The median time to onset of select adverse events ranged from 5 weeks for skin problems to 15 weeks for renal effects. About one-quarter of patients received systemic immune-modulating agents to manage select adverse events, which resolved in most cases.
Select adverse events are those considered to be autoimmune related. Patients who developed three or more select adverse events had superior ORR (overall response rate) than patients with 1 or 2 select adverse events, or no adverse events.
In all patients receiving nivolumab monotherapy (N = 576), the ORR was 31.4%, and median PFS was 4.7 months (95% CI, 3.4 to 5.6). In a multivariable analysis…ORR was significantly better in patients who experienced treatment-related select AEs of any grade compared with those who did not, with greater benefit in patients who reported three or more or one to two treatment-related select AEs, compared with those with none. In contrast, there was no significant difference in ORR on the basis of the occurrence of grade 3 to 4 treatment-related select AEs.
The administration of immunomodulatory drugs to treat select adverse events did not diminish response.
