Two new companies have received substantial funding to pursue neo-antigens for cancer immunotherapy: Gritstone Oncology raised $102MM to pursue lung cancer and Neon Therapeutics raised $55MM to develop neoantigen-based therapeutic vaccines and T cell therapies to treat cancer.
What are neoantigens?
Neoantigens result from mutations occurring during tumor growth, are recognized as foreign and differ from native antigens to which the immune system is tolerant. Mounting evidence suggests that immune rejection of tumors, for example that which is seen with checkpoint modulators, may be mediated by recognition of neoantigens.
The clinical relevance of T cells in the control of a diverse set of human cancers is now beyond doubt. However, the nature of the antigens that allow the immune system to distinguish cancer cells from non-cancer cells has long remained obscure. Recent technological innovations have made it possible to dissect the immune response to patient-specific neoantigens that arise as a consequence of tumor-specific mutations, and emerging data suggest that recognition of such neoantigens is a major factor in the activity of clinical immunotherapies. These observations indicate that neoantigen load may form a biomarker in cancer immunotherapy and provide an incentive for the development of novel therapeutic approaches that selectively enhance T cell reactivity against this class of antigens.
How was the significance of neoantigens in cancer identified?
The inspiration to form Gritstone came from a paper that focused on the higher likelihood of a patient’s response to the checkpoint inhibitor Yervoy (ipilimumab, a monoclonal antibody that disrupts the CTLA-4/ checkpoint blockade) based on the proliferation of tumor neoantigens.
Malignant melanoma exomes from 64 patients treated with CTLA-4 blockade were characterized with the use of massively parallel sequencing. A discovery set consisted of 11 patients who derived a long-term clinical benefit and 14 patients who derived a minimal benefit or no benefit. Mutational load was associated with the degree of clinical benefit (P=0.01) but alone was not sufficient to predict benefit. Using genomewide somatic neoepitope analysis and patient-specific HLA typing, we identified candidate tumor neoantigens for each patient. We elucidated a neoantigen landscape that is specifically present in tumors with a strong response to CTLA-4 blockade. We validated this signature in a second set of 39 patients with melanoma who were treated with anti-CTLA-4 antibodies. Predicted neoantigens activated T cells from the patients treated with ipilimumab.
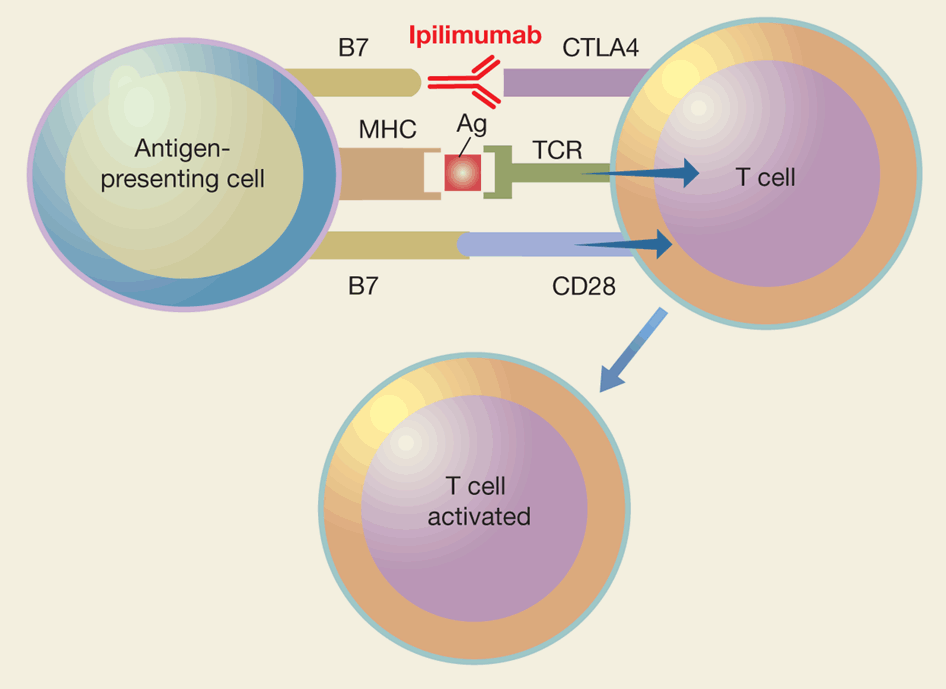
Ipilimumab stimulates antitumor immunity by blocking CTLA4, a natural brake on T cells, and allowing their unimpeded ‘costimulation’. http://www.nature.com/nbt/journal/v28/n8/fig_tab/nbt0810-763_ft.html
Following treatment with ipilimumab, CD8+ T-cell responses against GLEREGFTF a mutated peptide fragment of CSMD1 (a protein phosphatase) of was seen, but not to the unmutated peptide fragment GLERGGFTF. This demonstrates that mutations in cancer proteins offer non-self antigenic targets for immune attack.
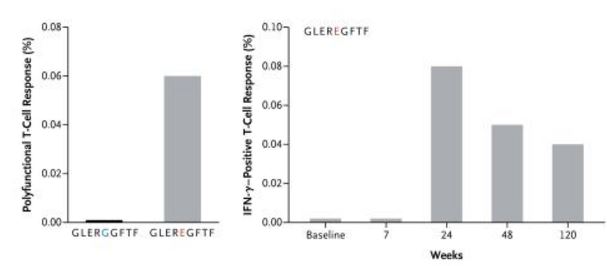
This shows the dual positive (IFN-γ and TNF-α) CD8+ T-cell response to GLEREGFTF and nonmutant peptide GLERGGFTF and illustrates the increase in peptide-specific T cells 24 weeks after the initiation of treatment with ipilimumab relative to baseline.
Neoantigens in lung cancer
The importance of neoantigens in lung cancer was demonstrated in another study in which anti-PD-1 antibody pembrolizumab (Keytruda) was administered.
Immune checkpoint inhibitors, which unleash a patient’s own T cells to kill tumors, are revolutionizing cancer treatment. To unravel the genomic determinants of response to this therapy, we used whole-exome sequencing of non–small cell lung cancers treated with pembrolizumab, an antibody targeting programmed cell death-1 (PD-1). In two independent cohorts, higher nonsynonymous mutation burden in tumors associated with improved objective response, durable clinical benefit, and progression-free survival. Efficacy also correlated with the molecular smoking signature, higher neoantigen burden, and DNA repair pathway mutations; each factor was also associated with mutation burden. In one responder, neoantigen-specific CD8+ T cell responses paralleled tumor regression, suggesting that anti-PD-1 therapy enhances neoantigen-specific T cell reactivity. Our results suggest that the genomic landscape of lung cancers shape response to anti-PD-1 therapy.
Since cancers, especially invasive cancers, have amassed sufficient mutations to block the expression of DNA repair and apoptosis genes, they accumulate mutations at a high rate. These mutations make for a rich source of novel antigens for the immune system to recognize and attack. In fact, in patients with advanced carcinomas that have mismatch repair deficiencies, greater efficacy of immune checkpoint inhibition with pembrolizumab was observed than in patients with cancers in which mismatch repair deficiency was not present:
The immune-related objective response rate and immune-related progression-free survival rate were 40% (4 of 10 patients) and 78% (7 of 9 patients), respectively, for mismatch repair-deficient colorectal cancers and 0% (0 of 18 patients) and 11% (2 of 18 patients) for mismatch repair-proficient colorectal cancers. The median progression-free survival and overall survival were not reached in the cohort with mismatch repair-deficient colorectal cancer but were 2.2 and 5.0 months, respectively, in the cohort with mismatch repair-proficient colorectal cancer (hazard ratio for disease progression or death, 0.10 [P<0.001], and hazard ratio for death, 0.22 [P=0.05]). Patients with mismatch repair-deficient noncolorectal cancer had responses similar to those of patients with mismatch repair-deficient colorectal cancer (immune-related objective response rate, 71% [5 of 7 patients]; immune-related progression-free survival rate, 67% [4 of 6 patients]). Whole-exome sequencing revealed a mean of 1782 somatic mutations per tumor in mismatch repair-deficient tumors, as compared with 73 in mismatch repair-proficient tumors (P=0.007), and high somatic mutation loads were associated with prolonged progression-free survival (P=0.02).
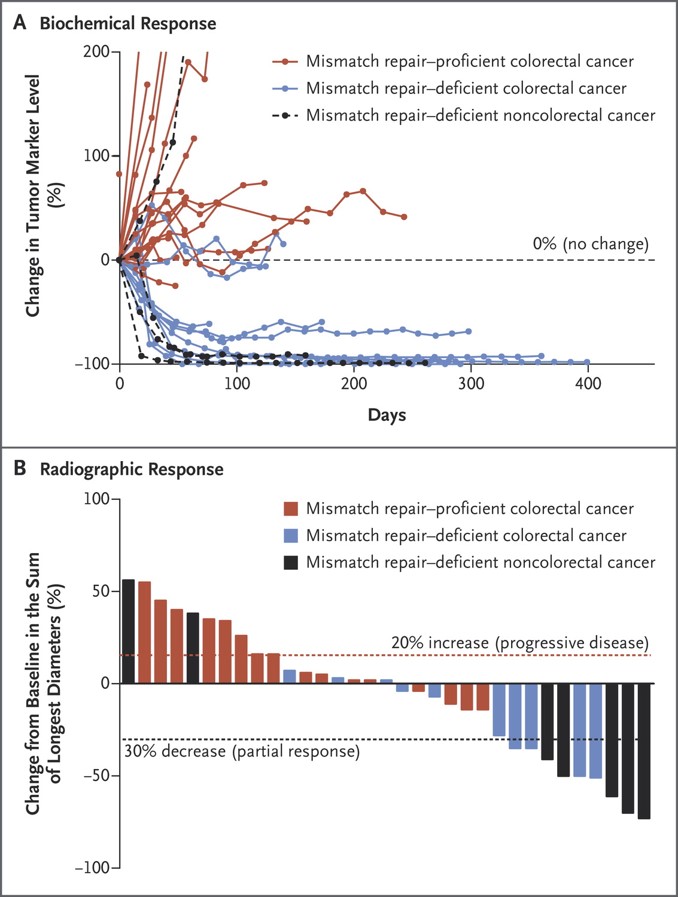
Clinical Responses to Pembrolizumab Treatment. The biochemical responses to pembrolizumab treatment are shown in Panel A. Serum levels of protein biomarkers were measured at the start of each treatment cycle, and the values represent percentage changes from baseline. Each line represents one patient; patients were included if their baseline tumor marker values were higher than the upper limit of normal. CA-125 was used as the biomarker for one patient with endometrial cancer, CA19-9 was used for one patient with cholangiocarcinoma and one patient with ampullary cancer, and carcinoembryonic antigen (CEA) was used for all other patients. Radiographic responses to treatment with pembrolizumab, evaluated on the basis of Response Evaluation Criteria in Solid Tumors (RECIST), are shown in Panel B. Tumor responses were measured at regular intervals, and the values shown are the largest percentage change in the sum of longest diameters from the baseline measurements of each measurable tumor. Each bar represents one patient. Le DT et al. N Engl J Med 2015;372:2509-2520.
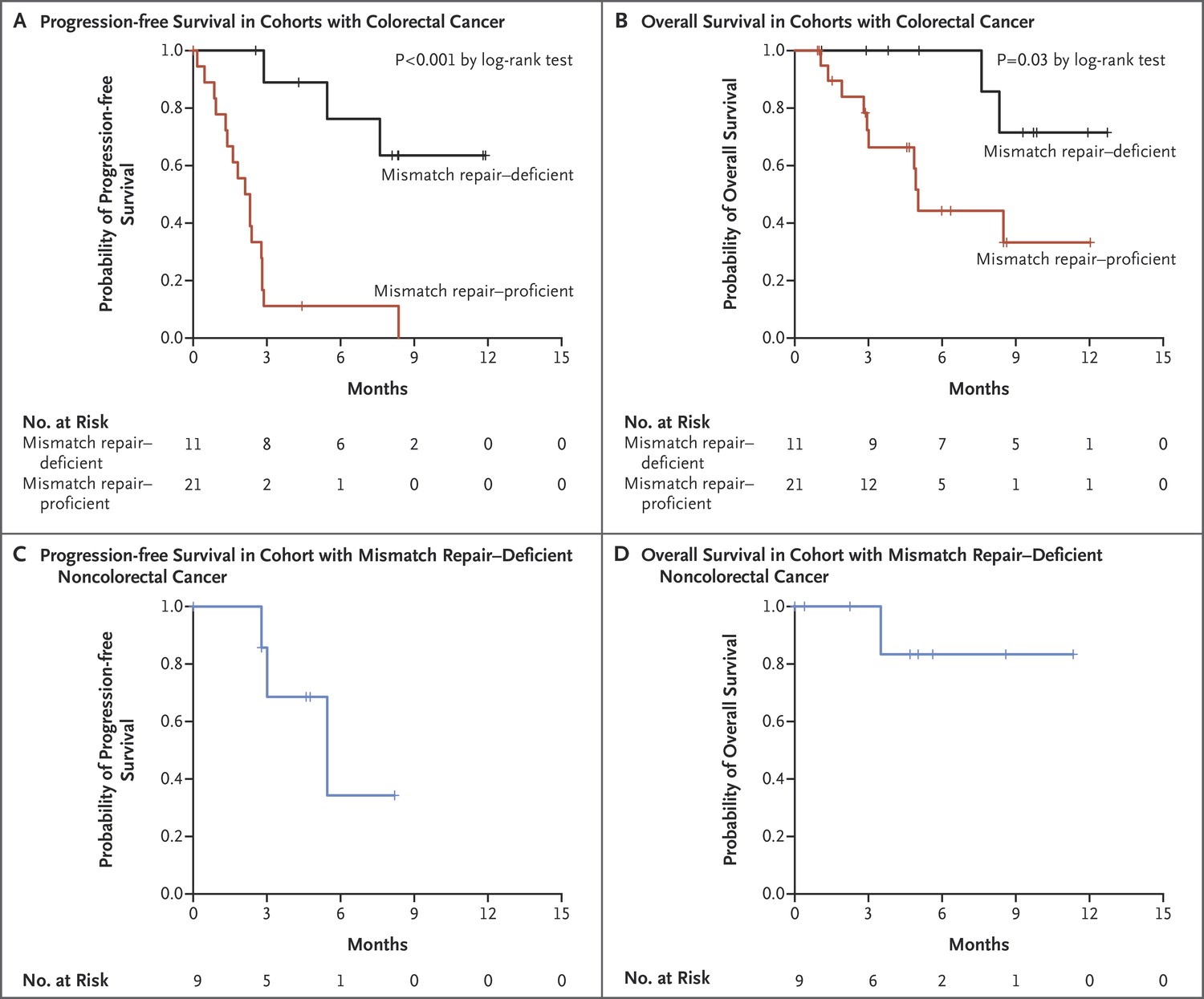
Clinical Benefit of Pembrolizumab Treatment According to Mismatch-Repair Status. Kaplan–Meier curves are shown for progression-free survival in the cohorts with colorectal cancer (Panel A), overall survival in the cohorts with colorectal cancer (Panel B), progression-free survival among patients with mismatch repair–deficient noncolorectal cancers (Panel C), and overall survival among patients with mismatch repair–deficient noncolorectal cancers (Panel D). In both cohorts with mismatch repair–deficient tumors, median overall survival was not reached. Patients in the cohort with mismatch repair–proficient cancers had a median progression-free survival of 2.2 months (95% CI, 1.4 to 2.8) and a median overall survival of 5.0 months (95% CI, 3.0 to not estimable). Patients with mismatch repair–deficient noncolorectal cancers had a median progression-free survival of 5.4 months (95% CI, 3 to not estimable). Le DT et al. N Engl J Med 2015;372:2509-2520.
Gritstone will take tumor samples from newly diagnosed lung cancer patients, run them through DNA sequencing machines to look for specific mutations, and then select the most important markers that could serve as antigens to the immune system. Those antigens are elusive. Scientists know tumors have a way of mutating over time, creating all sorts of odd markers that the immune system may or may not recognize as worthy of destruction. Once the patient’s specific “neoantigens” are selected, they’ll be incorporated into a vaccine. Patients will still likely get anti-PD1 antibodies (Opdivo or Keytruda).

