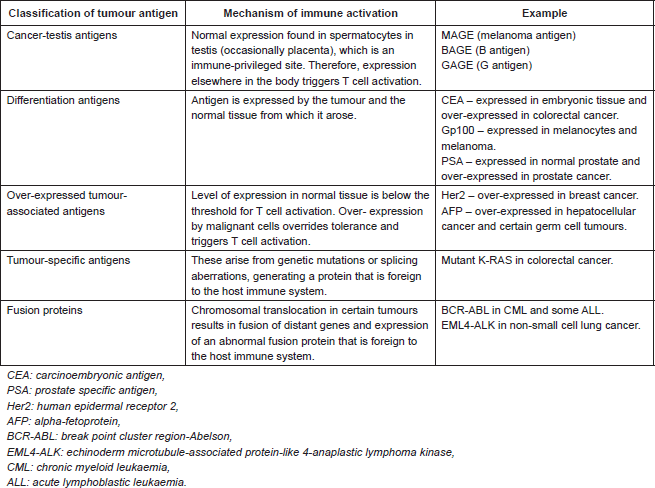
ImaginAB and Eli Lilly announced a pre-clinical collaboration to use ImaginAb’s immune imaging agent IAB22M2C to study new T-cell therapies cancer.
IAB22M2C is a PET-based imaging agent that flags CD8-positive T cells, helping capture a snapshot of a patient’s immune response that can be used for patient selection and an improved understanding of the potential impact of immuno-oncology drugs.
Why is it important to know about CD8+ Cells in vivo?
CD8+ T-cells (Cytotoxic T-cells or CTL’s) are the main effectors of the cell-mediated attack that the body initiates on cancer. Cancers evade destruction when a suboptimal CD8+ T-cell response is mounted (or when no CD8+ response is generated), and when immune checkpoints abrogate the activity of CD8+ cells that have been generated. For checkpoint inhibitors to work, an adequate first wave immune response needs to have been generated, that is, CD8+ T-cell response must have been initiated by successful antigen presentation to CD4+ cells, which in turn, stimulate antigen-specific CD8+ CTL responses.
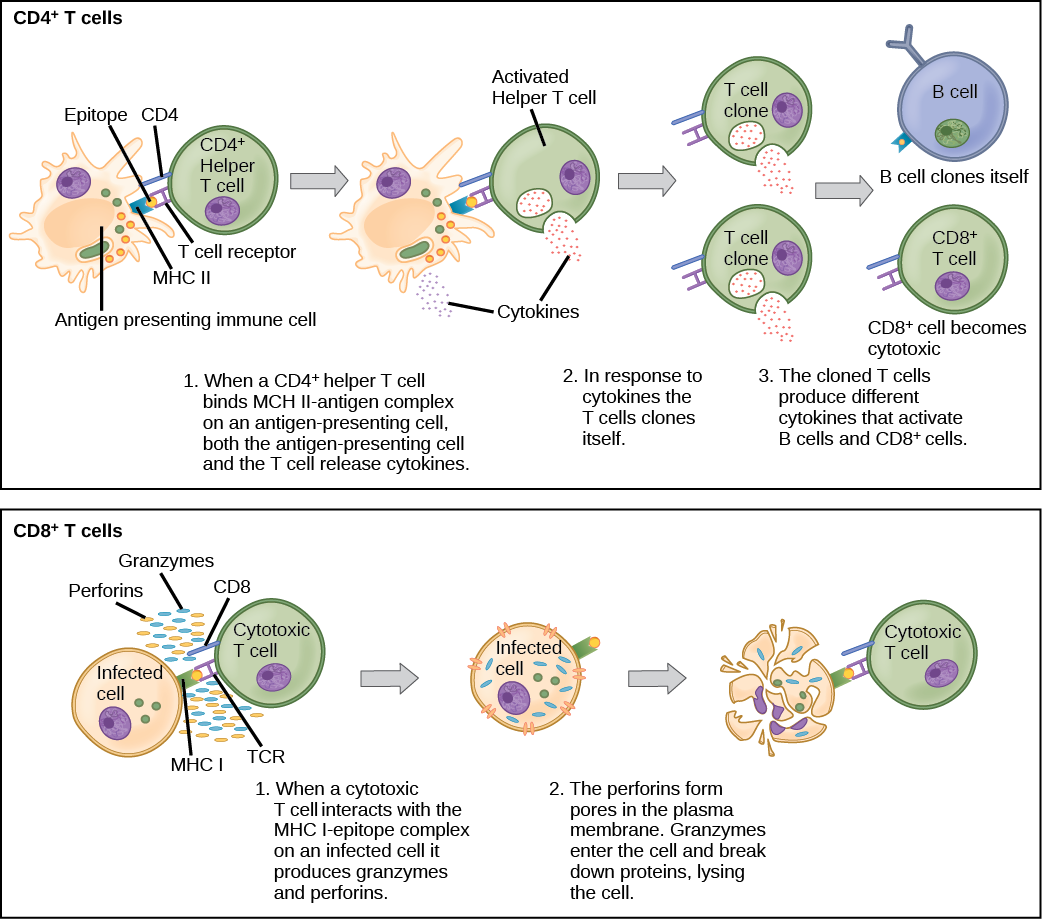
Naïve CD4+ T cells engage MHC II molecules on antigen-presenting cells (APCs) and become activated. Clones of the activated helper T cell, in turn, activate B cells and CD8+ T cells, which become cytotoxic T cells. Cytotoxic T cells kill infected cells. http://philschatz.com/biology-book/contents/m44821.html
What if an inadequate CD8+ T-cell response is observed?
ImaginAB’s PET imaging product can help determine whether CD8+ T-cells that localize to cancer sites have been induced. If not, strategies to initiate an adequate CD8+ response against cancer antigens, for example, using cancer vaccines (i.e., whole cancer cells, dendritic cells, cancer antigen proteins or peptides, viruses, and DNA), are needed.
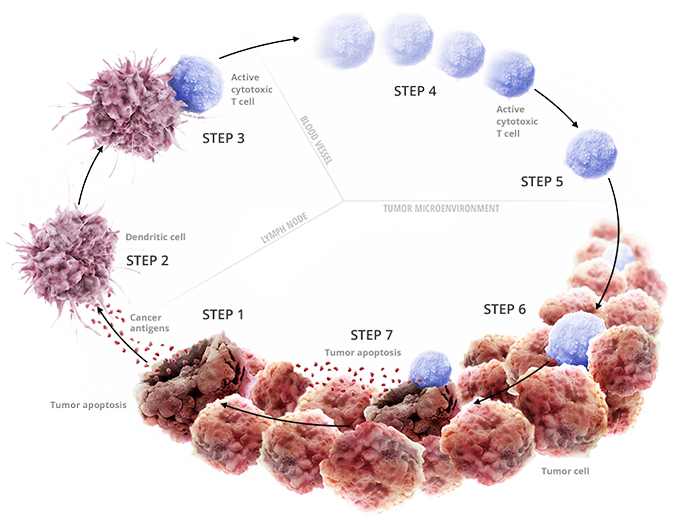
STEPS 1-3: INITIATING AND PROPAGATING ANTICANCER IMMUNITY
• Oncogenesis leads to the expression of neoantigens that can be captured by dendritic cells
• Dendritic cells can present antigens to T cells, priming and activating cytotoxic T cells to attack the cancer cells
STEPS 4-5: ACCESSING THE TUMOR
• Activated T cells travel to the tumor and infiltrate the tumor microenvironment
STEPS 6-7: CANCER-CELL RECOGNITION AND INITIATION OF CYTOTOXICITY
• Activated T cells can recognize and kill target cancer cells
• Dying cancer cells release additional cancer antigens, propagating the cancer immunity cycle
http://www.researchcancerimmunotherapy.com/overview/cancer-immunity-cycle
Augmenting response when CD8+ T-cell responses are seen
If CD8+ T-cells (CTL’s) that localize to sites of cancer are present, then checkpoint inhibition (e.g., PD-1 and PD-L1 inhibitors) strategies are warranted.
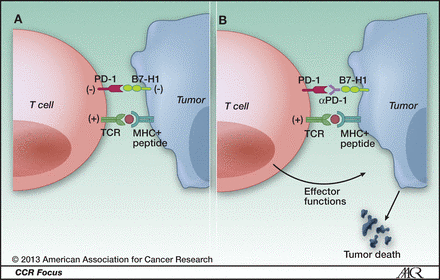
PD-1 and cancer. A, ligation of T-cell PD-1 by tumor B7-H1 results in the downregulation of T-cell effector functions that destroy tumor tissue. B, blockade of this pathway by anti-PD-1 antibodies prevents this downregulation, and allows T cells to maintain their antitumor functionality and ability to mediate tumor cell death. http://clincancerres.aacrjournals.org/content/19/5/1021.figures-only
ImaginAB’s Approach
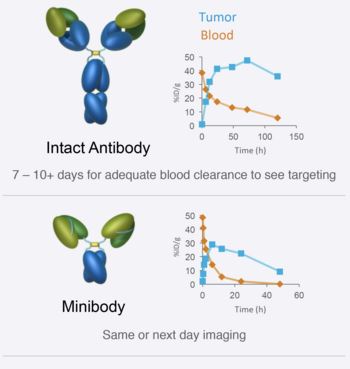
Minibodies are created from antibodies that bind to the targets of interest. They are engineered to improve pharmacokinetics, tissue localization, elimination, and to have an improved safety profile compared to traditional antibodies. This is achieved by re-engineering intact antibodies into smaller protein formats that have significant advantages for imaging:
- Inert /nonfunctional – antibody effector functions and FcRN binding regions are removed
- Non-glycosylated so protein heterogeneity is minimized
- Faster clearance
- Superior tissue penetration
- Steerable organ of clearance
“Selecting the proper patients for immunotherapy continues to be a major challenge for the new wave of cancer therapies coming to market,” said Dr. Roger Crystal, chief business officer for ImaginAb. “Same-day CD8 imaging holds tremendous potential in helping to guide treatment for cancer immunotherapies, and we look forward to pioneering this approach with Lilly.”