Bristol Myers Squibb (BMS) and Nektar Therapeutics announced a collaboration in which BMS’ PD-1 checkpoint inhibitor (Opdivo, nivolumab) will be combined with Nektar;s CD-122 agonist NKTR-214.
Opdivo, a monoclonal antibody, works by binding to PD-1 expressed on T-cells, thereby inhibiting its interaction with PD-L1, which is expressed on cancer cells. Blocking this interaction protects cytotoxic T-cells from dying, allowing them to attack the cancer (Figure 1).
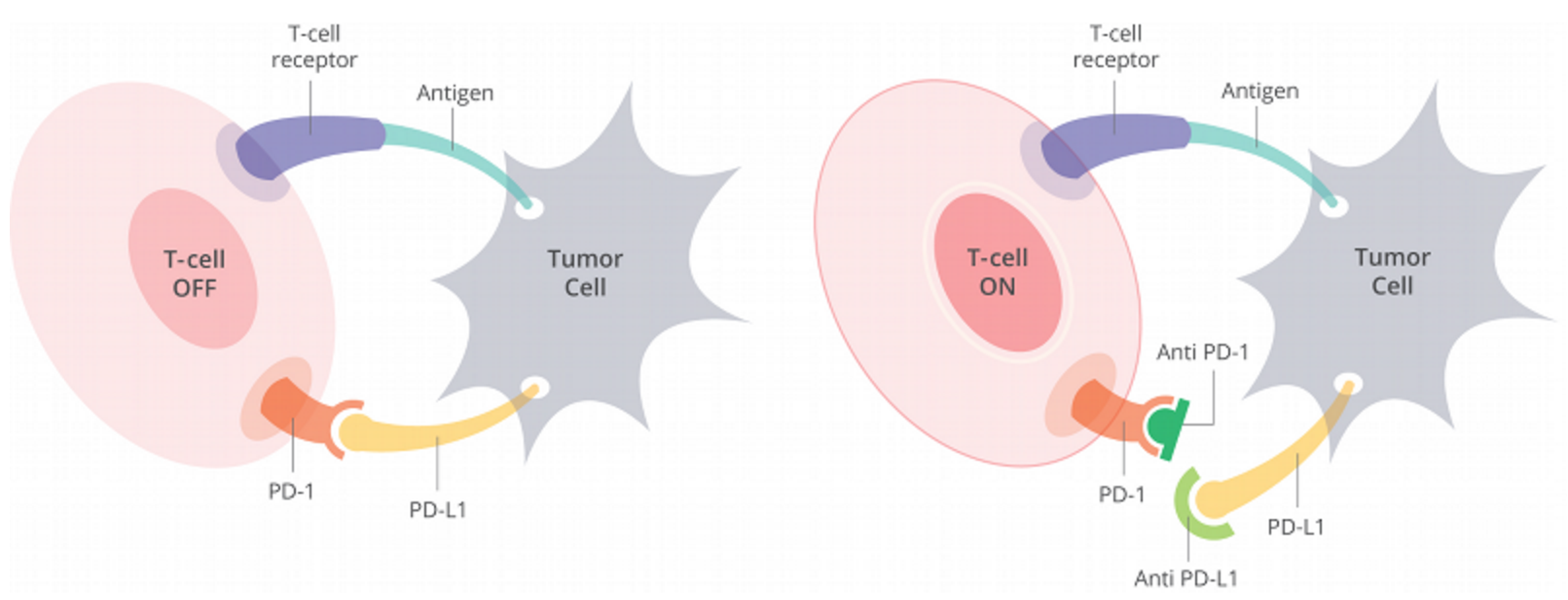
Figure 1. Cells have a protein on their surface called PD-1 (in orange above). When PD-1 binds to PD-L1 (yellow) on another cell, the T cell becomes deactivated. Most cancer cells have PD-L1 on their surface and escape being killed by turning off the T cell in this way. Anti-PD-1 antibodies (dark green) or anti-PD-L1 antibodies (light green) can prevent the tumor cell from binding PD-1 and thus allow T cells to remain active. http://www.curetoday.com/articles/fda-approves-frontline-opdivo-for-braf-mutant-melanoma
What is CD-122?
CD-122 is the beta subunit of the interleukin-2 (IL-2) receptor:
The interleukin 2 receptor, which is involved in T cell-mediated immune responses, is present in 3 forms with respect to ability to bind interleukin 2. The low affinity form is a monomer of the alpha subunit and is not involved in signal transduction. The intermediate affinity form consists of an alpha/beta subunit heterodimer, while the high affinity form consists of an alpha/beta/gamma subunit heterotrimer. Both the intermediate and high affinity forms of the receptor are involved in receptor-mediated endocytosis and transduction of mitogenic signals from interleukin 2. The protein encoded by CD-122 represents the beta subunit and is a type I membrane protein.
![Figure 2. IL-2 Receptor (IL-2R) Binding and Signaling. Cartoon of IL-2 interacting with its receptor subunits, including IL-2Rα (CD25), IL-2Rβ (CD122), and the common γ-chain (γc, CD132), as well as signaling pathways following the interaction of IL-2 with various IL-2R subunits. Binding of IL-2 to CD122 and γc causes heterodimerization of the cytoplasmic tails of these receptor subunits and activation of Janus kinase 1 (JAK1) and JAK3 (associated with CD122 and γc, respectively) [18]. Activated JAK1 and JAK3 exert kinase activity on key tyrosine (Y) residues of CD122, which subsequently allows recruitment of the adaptor protein SHC and of STAT1, STAT3, and STAT5 (including STAT5A and STAT5B). Phosphorylated STAT5A and STAT5B then oligomerize forming STAT5 dimers and tetramers before undergoing nuclear translocation, where they bind to key target genes responsible for cell activation, differentiation, and proliferation. SHC in turn serves as a platform for activating the Ras–Raf–MEK–ERK mitogen-activated protein kinase (MAPK) pathway. Additionally, IL-2R triggering activates the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR)–p70 S6 kinase pathway. http://www.cell.com/trends/immunology/fulltext/S1471-4906(15)00248-3](http://blogs.shu.edu/cancer/files/2016/11/CD-122-receptor.jpg)
Figure 2. IL-2 Receptor (IL-2R) Binding and Signaling. Cartoon of IL-2 interacting with its receptor subunits, including IL-2Rα (CD25), IL-2Rβ (CD122), and the common γ-chain (γc, CD132), as well as signaling pathways following the interaction of IL-2 with various IL-2R subunits. Binding of IL-2 to CD122 and γc causes heterodimerization of the cytoplasmic tails of these receptor subunits and activation of Janus kinase 1 (JAK1) and JAK3 (associated with CD122 and γc, respectively) [18]. Activated JAK1 and JAK3 exert kinase activity on key tyrosine (Y) residues of CD122, which subsequently allows recruitment of the adaptor protein SHC and of STAT1, STAT3, and STAT5 (including STAT5A and STAT5B). Phosphorylated STAT5A and STAT5B then oligomerize forming STAT5 dimers and tetramers before undergoing nuclear translocation, where they bind to key target genes responsible for cell activation, differentiation, and proliferation. SHC in turn serves as a platform for activating the Ras–Raf–MEK–ERK mitogen-activated protein kinase (MAPK) pathway. Additionally, IL-2R triggering activates the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR)–p70 S6 kinase pathway. http://www.cell.com/trends/immunology/fulltext/S1471-4906(15)00248-3
IL-2 is a critically important cytokine in the homeostasis and activation of the immune system. It is a major cytokine involved in precipitating adaptive immune responses, stimulating T-helper (CD-4) and cytotoxic (CD-8+) – see Figure 3. However, IL-2 also maintains homeostasis by stimulating Treg cells, which suppress adaptive immune responses.
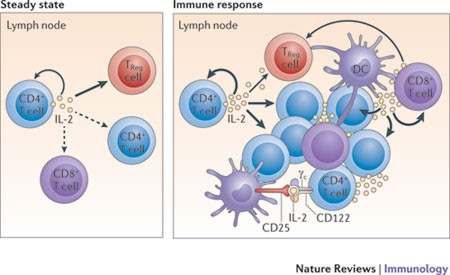
Figure 3. Under steady-state resting conditions, interleukin-2 (IL-2) is mainly produced by CD4+ T cells that are activated by foreign- and self-peptide–MHC class II complexes on dendritic cells (DCs; not shown) in secondary lymphoid organs, such as the lymph nodes. The secreted IL-2 is then consumed at the same site by CD25+ cells, notably regulatory T (TReg) cells, and also by adjacent activated CD4+ and CD8+ T cells. During an immune response, activated DCs home to the draining lymph nodes, where activated CD4+ and CD8+ T cells produce large amounts of IL-2. IL-2 is then consumed by CD25+ effector T cells and TReg cells. Activated DCs express CD25 on their cell surface; such CD25 molecules might bind to either T cell- or DC-derived IL-2 for trans-presentation to neighbouring CD25low effector CD4+ T cells (and perhaps also CD8+ T cells) early during T cell activation, before the T cells express high levels of CD25. γc, common cytokine receptor γ-chain. http://www.nature.com/nri/journal/v12/n3/fig_tab/nri3156_F1.html
IL-2 is approved for the treatment of patients with renal cancer (RCC) and melanoma; both tumors are immunologically modulated cancers, demonstrated by spontaneous regression of patients with advanced disease who were not receiving any systemic treatment. High does therapy with IL-2 is very beneficial to patients with RCC and melanoma (Figure 4).
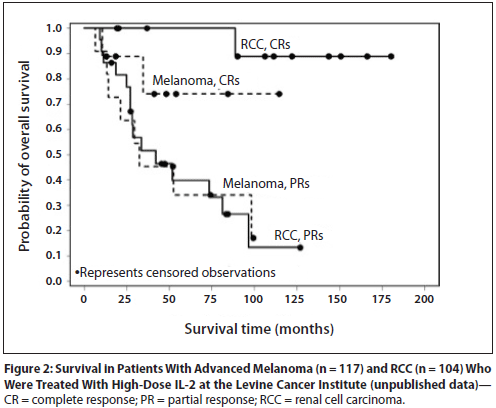
Figure 4. Survival in patients with advanced melanoma (n = 117) and renal cell carcinoma (RCC, n = 104). Levince Cancer Institute – unpublished data. http://www.cancernetwork.com/oncology-journal/high-dose-interleukin-2-it-still-indicated-melanoma-and-rcc-era-targeted-therapies
What is NKTR-214?
NKTR-214 is an agonist of CD-122 that stimulates cytotoxic T-cells (CD-8+) and NK (natural killer cells). It is IL-2 conjugated with polyethylene glycol (PEG) to mask the region of IL-2 that interacts with the IL2Rα subunit responsible for activating immunosuppressive Tregs (regulatory T-cells), biasing activity towards tumor killing CD8+ T cells. NKTR-214 is a biologic prodrug consisting of IL2 bound by 6 releasable polyethylene glycol (PEG) chains (Figure 5).
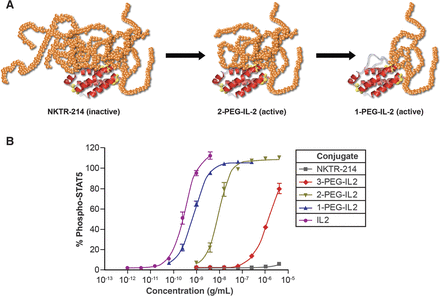
Figure 5. A, schematic of the inactive NKTR-214 prodrug releasing to IL2 conjugates bound by fewer PEG chains with increasing bioactivity. Red depicts the IL2 cytokine core, and orange depicts the polymer chains located at the IL2/IL2Rα interface. B, identification of the most active IL2 conjugates created by hydrolysis of NKTR-214 as measured by phosphorylation of STAT5 in murine CTLL-2 T cells. Purified forms of the released conjugated IL2 forms were assayed. On the basis of the EC50, the 2-PEG-IL2 and 1-PEG-IL2 derived from NKTR-214 are identified as the most active conjugates released from NKTR-214. EC50 values for STAT5 phosphorylation were determined from two or more independent experiments, each performed in triplicate. The IL2 control is a purified reference standard. http://clincancerres.aacrjournals.org/content/22/3/680
In preclinical studies, NKTR-214 demonstrated a mean ratio of 450:1 within the tumor micro-environment of CD8-positive effector T cells, which promote tumor destruction, compared with CD4-positive regulatory T cells, which are a type of cell that can suppress tumor-killing T cells.2 Furthermore, a single dose of NKTR-214 resulted in a 500-fold AUC exposure within the tumor compared with an equivalent dose of the existing IL-2 therapy, enabling, an antibody-like dosing regimen for a cytokine. In dosing studies in non-human primates, there was no evidence of severe side effects such as low blood pressure or vascular leak syndrome with NKTR-214 at predicted clinical therapeutic doses.
Clinical trials of NKTR-214
A Phase 1/2 Dose Escalation and Expansion Study Of NKTR-214 In Subjects With Locally Advanced Or Metastatic Solid Tumors (NKTR-214) is currently recruiting patients.
This is a first in human, open-label, sequential dose escalation and expansion Phase 1/2 study of NKTR- 214 in adult patients with locally advanced and metastatic solid tumors. The Phase 1 stage of the study is designed as an open-label dose escalation trial of NKTR-214 in participants with locally advanced or metastatic solid tumors. The goal of the dose escalation stage of the study is to find the recommended phase 2 dose, to evaluate the efficacy of NKTR-214 by assessing the objective response rate and to evaluate the safety of NKTR-214. Immunological biomarkers in plasma and tumor samples will also be measured. Following this Phase 1 stage of the study, the Phase 2 expansion stage of the study will evaluate NKTR-214 in specific tumor settings.
In addition to this, Nektar Therapeutics has entered into a Phase 1/2 clinical research collaboration with The University of Texas MD Anderson Cancer Center to evaluate NKTR-214 in a variety of tumor types as a monotherapy and in combination with checkpoint inhibitors such as anti-PD-1 (Opdivo, nivolumab) and anti-CTLA-4 (Yervoy, iplilimumab).
Rationale for combining nivolumab and NKTR-214
There is sound rationale for combining NKTR-214 and checkpoint inhibitors – the former has the potential of accelerating the CD8+ specific immune response against the tumor (without stimulating Treg cells, which suppress CD8+ cells) and the latter can prevent abrogation of the immune response, enabling stronger immune attack for a longer period. That, of course, is the hope.

I am an oncologist in Puerto Rico and I am interested in running a clinical trial of NKTR 214 plus Opdivo in Triple Negative Breast Cancer ir other solid tumors. Please contact me at raul@nairesearch.org or 787-354-0758.
Thanks,
Raúl H. Morales-Borges, MD
Hi. Sorry, we don’t produce reprints, but feel free to do so, provided that you reference the blog post and the sources of information and diagrams/figures.
Do you have a reprint of this that you could share?