Genspera is a company developing novel prodrugs for the treatment of cancer. In a phase 2 study in patients with hepatocellular carcinoma who have failed treatment with Nexavar (sorafenib), a tyrosine kinase inhibitor that blocks KIT, FLT-3 RET, raf, VEGFR1-3, and PDGF kinases, a doubling of time to progression was seen in patients taking G-202.
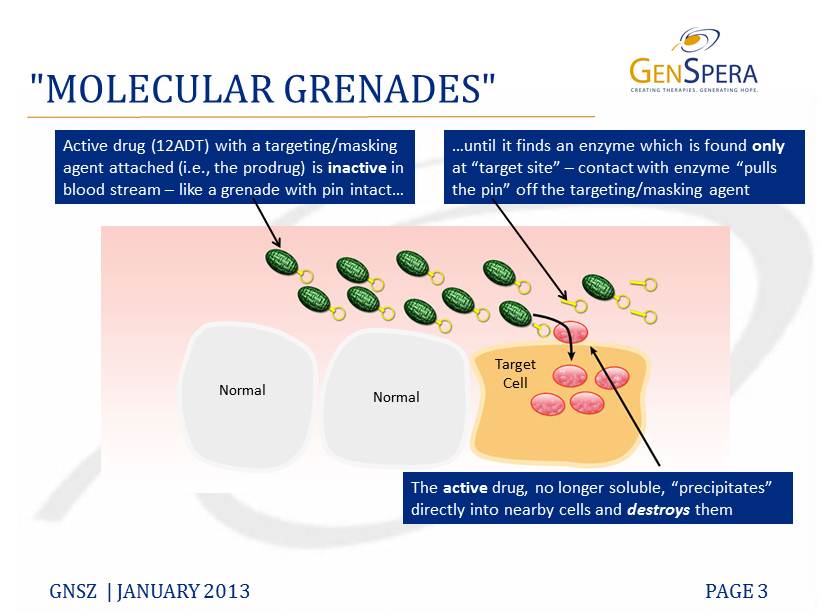
How does G202 work? The active moiety is thapsigargin (12ADT), a plant-derived toxin that shuts down Ca++ pumps in the endolasmic reticulum, which leads to apoptosis and cell death. 12ADT is chemically joined to a peptide that makes 12ADT more soluble and INACTIVE. When the prodrug encounters the appropriate enzymes that cleave the peptide from 12ADT, the toxin is released and taken-up by cells, which then die.
PSMA – prostate specific membrane antigen, is an enzyme that is over-expressed on many cancer, as well as newly formed cancer blood vessels. (We have talked about PSMA previously on this blog as a target for nano-particles containing docetaxel.) It cleaves the bond between ADT12 and the peptide. The anti-tumor effects are both indirect (killing of the tumor neovasculature) and direct (killing of cancer cells). PSMA basically pulls the pin out of the hand grenade. (See diagram from Genspera SEC 8K filing.)
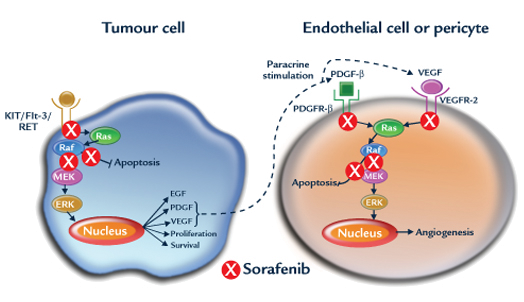
Cancer cells become resistant to Nexavar over time. Nexavar is a multi-kinase inhibitor targeting growth and proliferation pathways of the cancer cell and in the tumor vasculature. So, it, too, has direct tumoricidal and indirect (neovasculature) modes of action. FLT-3 activates both PI3K (Akt/PKB) and ras/raf pathways, thereby stimulating proliferation and inhibiting apoptosis of the cancer cell. Endothelial cells and pericytes possess VEGF and PDGF receptors, whose tyrosine kinases are also bocked by Nexavar.
The data from the Phase 2 G-202 study are impressive. Dr. Mahalingam, an oncologist at the Cancer Therapy & Research Center at the University of Texas Health Science Center at San Antonio, presented interim results from the Phase Ib and ongoing Phase II study in hepatocellular carcinoma (HCC) patients who had previously progressed on, or who were intolerant of, sorafenib. Historically, this patient population has a median time to progression of only two months when they enter subsequent clinical trials. Impressively, 80% of patients treated with G-202 had stable disease (no tumor growth) at two months and 50% of patients exhibited stable disease at 4 months on study.
“The efficacy and safety analyses on patients enrolled to date on this study continue to demonstrate that G-202 holds promise for patients with advanced HCC, with half the patients showing disease stability at 4 months and the majority of patients tolerating G-202 with minimal toxicities,” stated Dr. Mahalingam.