Last week, BIND Therapeutics and Amgen ended a collaboration on a targeted nanaoparticle that employed a proprietary cytotoxic compound from Amgen. However, BIND has several other programs (and partnerships with AstraZeneca, Roche, and Pfizer) that are proceeding in the clinic, which employ its Targeted Nanoparticle (TNP) technology. How does this work?
The goal of targeted nanoparticles is to deliver “payloads” (chemotherapeutics or toxins) to cancer cells with greater efficacy (cancer killing) and less toxicity. There are three primary considerations when designing a TNP:
- Targeting to a cancer antigen that is specific to cancer or over-expressed on cancer cells
- Escaping immune destruction of the TNP particle
- Delivery of the cytotoxic substance to the tumor cells at high concentrations
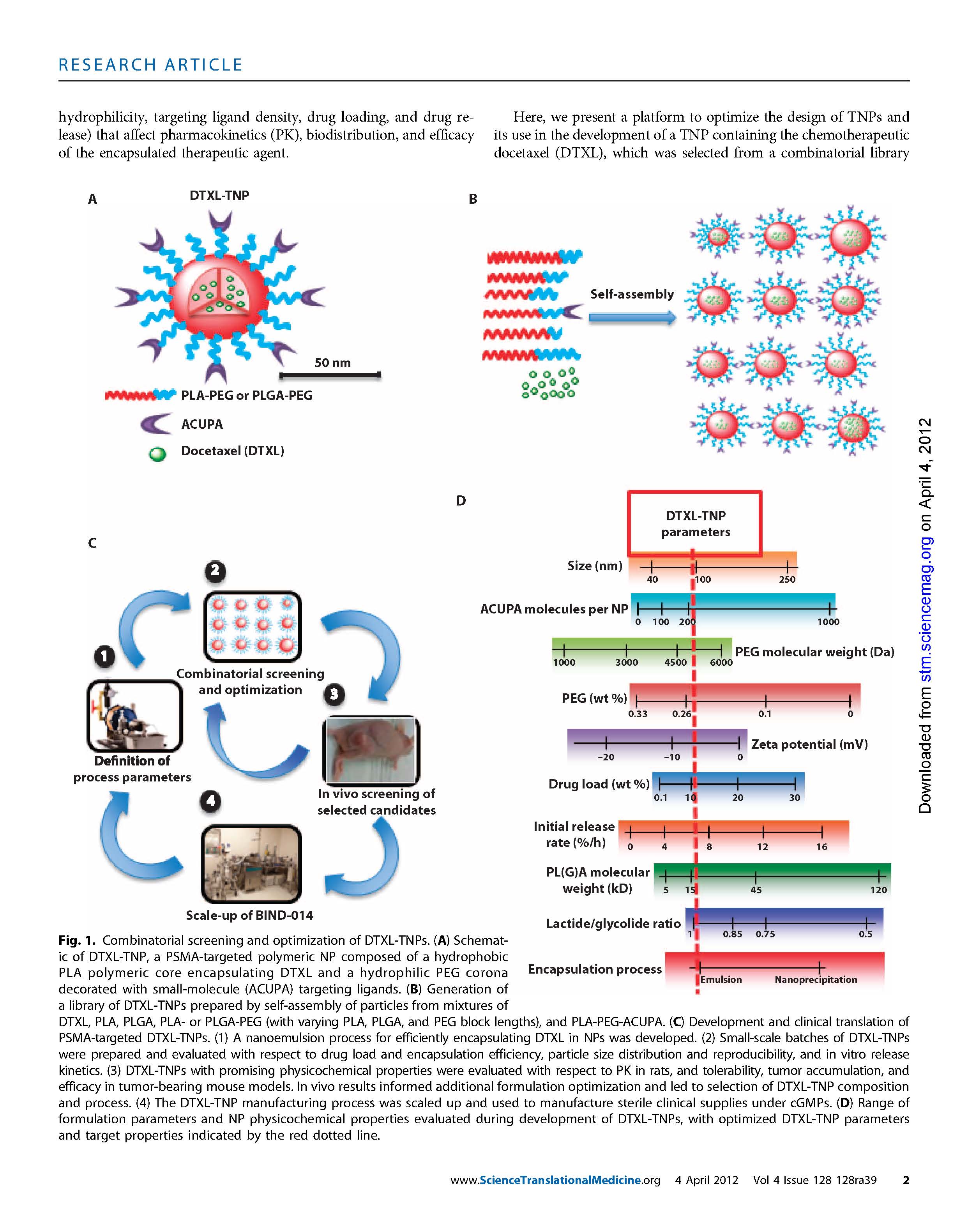
BIND’s prostate cancer product (BIND-014) employs a small molecule ligand (called ACUPA) that binds to the extracellular domain of the PSMA (Prostate Specific Membrane Antigen), a transmembrane glycoprotein that is over-expressed on prostate cancer cells and in tumor neo-vasculature. The chemotherapeutic compound, docetaxel, is contained within a biodegradable hydrophobic core that is used in liposomal drug delivery, e.g., PLA – poly-(DL-lactide) or PLGA – poly-(D,L-lactide-co-glycolide. [Docetaxel is FDA-approved for the treatment of prostate cancer, as well as breast, lung, gastric, and head and neck cancers.] Around the core is a hydrophilic PEG (poly-ethylene-glycol) corona to make the colecule immunologically inert and invisible to the immune system.
The trick is to optimize the molecule for enhanced tumor-penetrating properties, and pharmacokinetics (half-life in blood) to favor tumor accumulation, and tumor-killing pharmacodynamics. The physiochemical properties of the TNP govern its ability to circulate longer, accumulate in target tissues (cancer), and release the chemotherapeutic. Variables include the size of the molecule for extravasation through tumor vasculature, the number of molecules of docetaxel per hydrophobic core, the weight, the number of targeting molecules (ACUPA), etc. Optimization often requires trade-offs – area under the curve (pharmacokinetics of circulating drug) versus release efficiency of docetaxel. Because these properties can be “tuned” according to the physiochemical composition of the TNP, the technology is considered to be “programmable.” The resulting products are called Accurins.
The pre-clinical data looked very promising, with enhanced tumor uptake and tumor killing properties versus docetaxel, alone. BIND-014 has completed Phase 1 studies during which the MTD (maximum tolerate dose was established). Phase 2 studies have been initiated; it is during these studies that anti-tumor effects can be observed.