Great news – nivolumab, BMS’ PD-1 immune checkpoint control inhibitor was shown to prolong overall survival when used front-line in patients with advanced melanoma. The study was stopped early, that is before it fully enrolled the planned number of patients, by an independent data monitoring committee because it was shown to be quite effective.
Most exciting is that this study (called Checkmate-066) was in front-line usage, that is before the patient failed other systemic therapies. It was a randomized study comparing nivolumab versus the standard front-line therapy with dacarcazine, a DNA alkylating agent.
We have discussed immune checkpoint inhibitors before on this blog. PD-1 is a molecule expressed in the surface of T-cells, which when binding to PD-L1, causes the T-cell to down-regulate, that is, to no longer mount an anti-tumor immune response. This is a normal part of the second wave of cell mediated immunity. Nivolumab is an antibody that binds to PD-1 and blocks it from interacting with PD-L1 (expressed on cancer cells and antigen presenting cells), which then prevents Cytotoxic T-cells from becoming down-regulated against the antigens expressed on the neighboring TCR (T-cell receptor) – see article. Blocking a blocker allows the anti-tumor response (first wave) to proceed unabated, which in the context of cancer is the desired outcome.
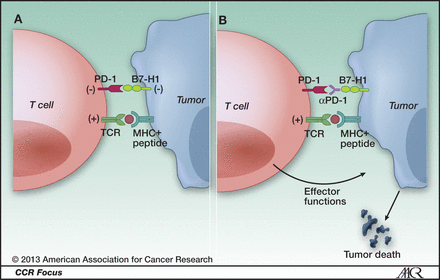
PD-1 and cancer. A, ligation of T-cell PD-1 by tumor B7-H1 results in the downregulation of T-cell effector functions that destroy tumor tissue. B, blockade of this pathway by anti-PD-1 antibodies prevents this downregulation, and allows T cells to maintain their antitumor functionality and ability to mediate tumor cell death.
