Vaccine strategies for cancer immunotherapy depend on the induction of dendritic cells (DC) in close proximity to T cells. This is best accomplished in lymphoid tissue. However, directing vaccines to lymphoid tissue has been quite challenging. RNA vaccines have emerged as a viable method of targeting systemic DCs with tumor-associated antigens. Further, RNA vaccines mimic viral infections, leading to synchronized induction of both highly potent adaptive as well as type-I-IFN-mediated innate immune mechanisms for cancer immunotherapy. The RNA constructs, themselves, apart from the antigenic epitopes or proteins for which they serve as the template for translation, serve as an immune adjuvant.
RNA Lipoplexes – RNA-LPX microparticles (LPX mRNA Vaccines)
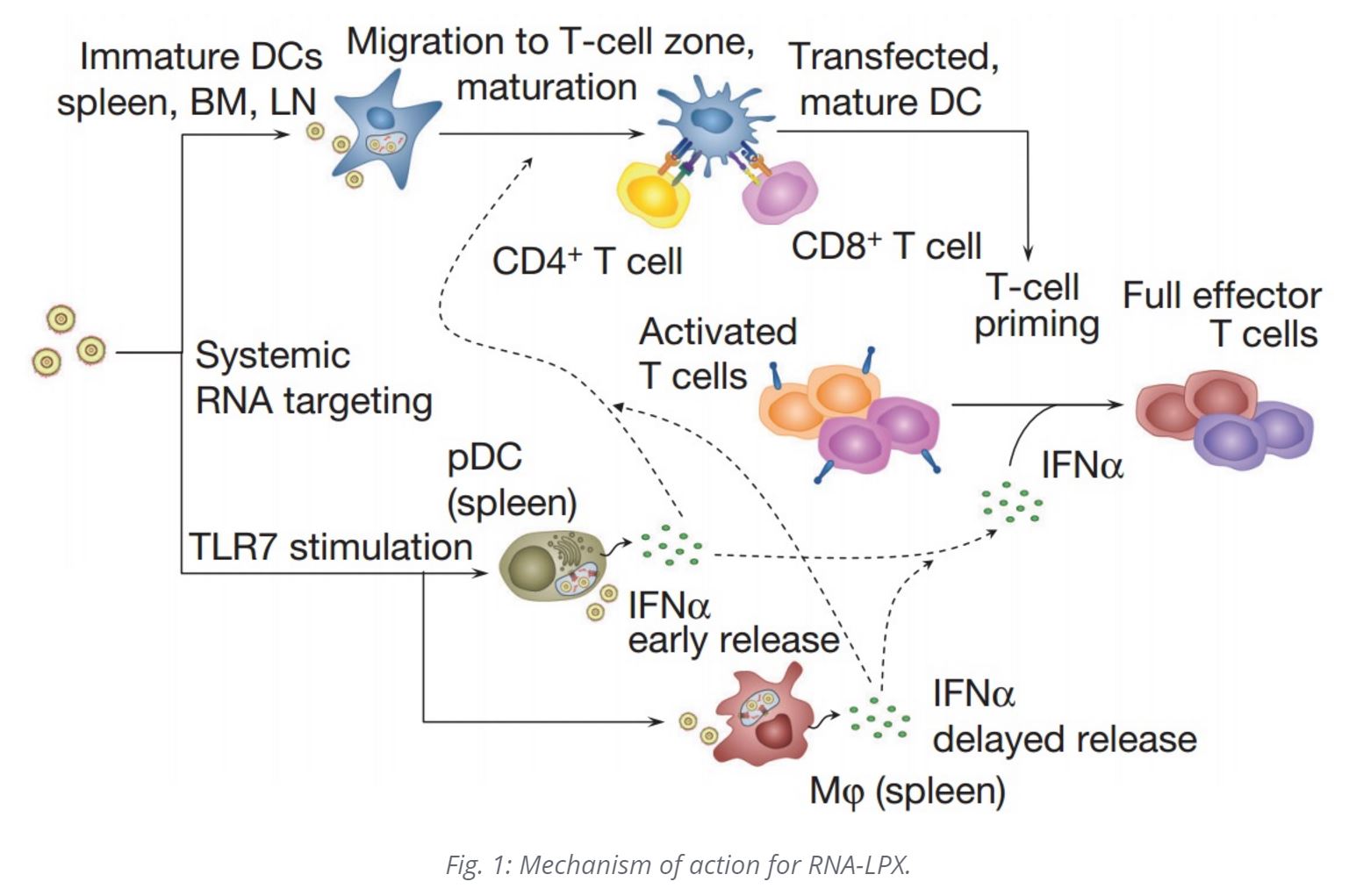
DCs engulf naked RNA injected into lymph nodes by macropinocytosis, which is constitutively active in immature DCs. Intravenously administered RNA-LPX nanoparticles taken up by monocyte-derived human immature DCs almost completely co-localized with the macropinosome marker dextran. This allows for the cancer antigens encoded in the mRNA to be translated and epitopes expressed on MHC Class I and II molecules of the dendritic cell, stimulating antigen-specific adaptive immune responses, including CTL (cytotoxic T-cell responses), as with viral infections.
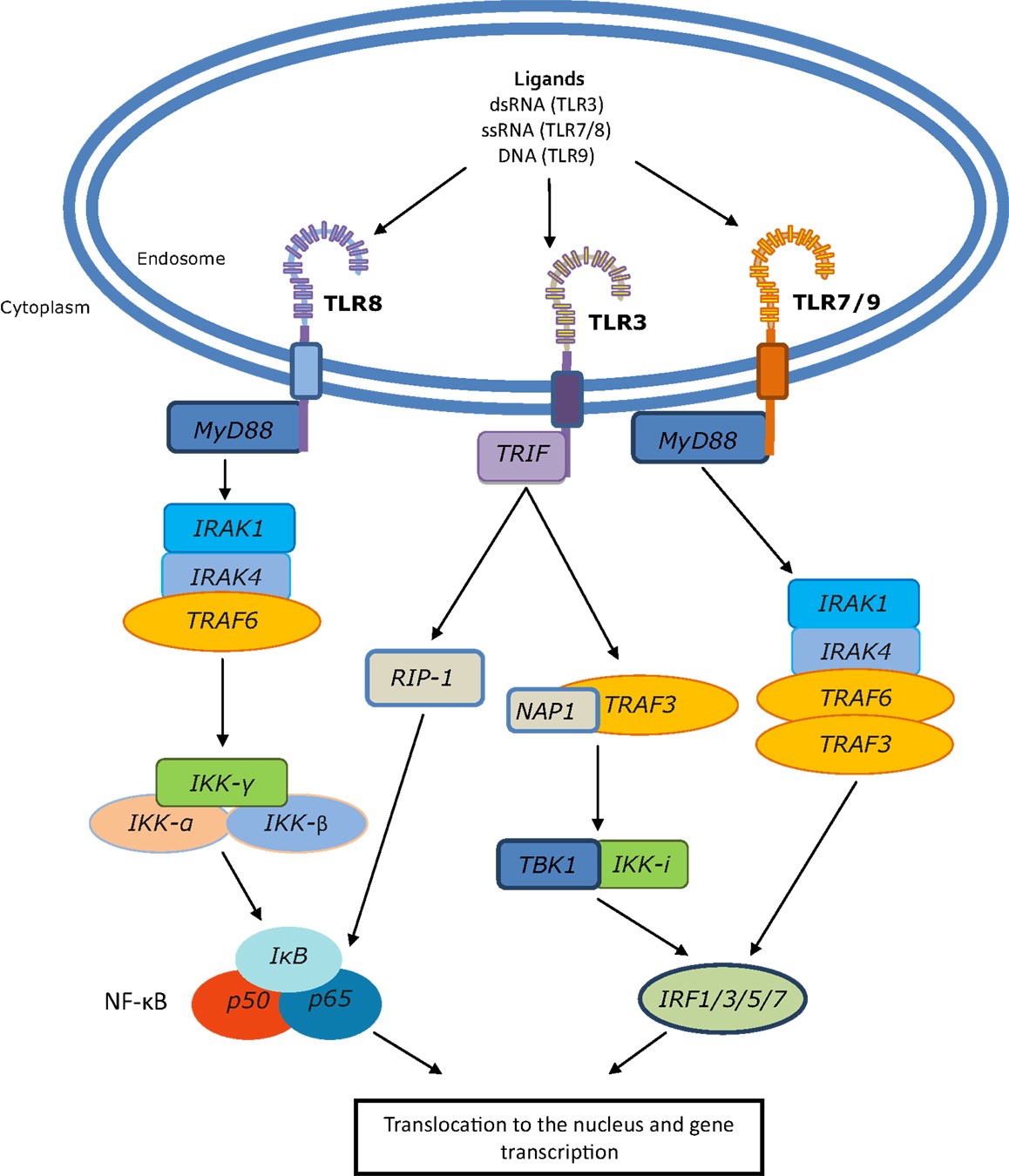
RNA-LPX nanoparticles also bind to Toll Like Receptor 3 and 7 (TLR3 and TLR7) in the endosomal compartment; this precipitates an anti-viral response from plasmacytoid dendritic cells (pDCs). IFNα is typically produced in the context of RNA virus infections by APCs (antigen presenting cells) sensing dsRNA and ssRNA via endosomal TLR3 and TLR7, respectively, and is crucial for an efficient inflammatory, antiviral environment. IFNα is required for the induction of antigen-specific (adaptive) immunity against viral infections.
Toll-like receptors (TLRs) are a family of evolutionarily conserved pattern recognition receptors. There are 10 human TLRs, which recognize pathogen associated molecular patterns from fungi, bacteria, and viruses, such as lipopolysaccharide (LPS), flagellin, and dsRNA. Most TLRs are expressed at the cell surface where they recognize mainly bacterial products, whereas TLRs 3, 7, 8, and 9 are localized to endosomal compartments and respond to self and foreign nucleic acid structures. TLR3 recognizes dsRNA, while TLR7 and TLR8 both respond to ssRNA. TLR9 recognizes DNA, the binding event of which was believed to be sequence specific, favoring CpG-rich DNA. However, recent evidence suggests that DNA conformation is actually the key mediator of TLR9 activation, not the DNA sequence. Localization of these TLRs to the endosome likely represents a method of sequestering these molecules from inadvertent activation by host nucleic acids.
Induction of TLR7-driven type-I interferon release is essential for the full anti-tumor potency of RNA-LPX vaccines. Type I IFN as a key molecule in antigen-specific immunity against viral infections has further effects, for example, upregulation of MHC expression, promotion of maturation and cross-presentation in DCs, and direct inhibition of regulatory T-cell functions. LPX mRNA vaccines connect effective cancer immunotherapy with host pathogen-defense mechanisms. Mechanisms of antiviral host defense are important for survival, conserved in all vertebrates and evolutionarily optimized for high sensitivity and potency. RNA-LPX vaccines appear to mimic infectious non-self and thus mobilize concomitantly adaptive and innate antiviral mechanisms. The i.v. delivery of RNA-LPX simulates a viraemic pathogen intrusion in the blood stream, and by reaching DCs in various lymphoid tissues, mobilizes the full T-cell repertoire for adaptive immune responses.
LPX RNA Vaccine in Melanoma
A phase I dose-escalation trial with RNA-LPX vaccines encoding four tumor antigens (NY-ESO-1, MAGE-A3, tyrosinase and TPTE – transmembrane phosphatase with tensin homology, a novel, 2761 bp long Cancer Testis Antigen located on chromosome 21p11 and shares significant homology with tumor suppressor protein PTEN) for intravenous administration is currently recruiting patients with advanced malignant melanoma (NCT02410733). The first three patients were treated with a very low initial dose of RNA-LPX, followed by four weekly applications with moderately higher doses, but still below the absolute therapeutic dose levels used in mice. All vaccine applications were well-tolerated with transient flu-like symptoms. All three patients had dose-dependent early release of IFNα and IP-10 (also known as CXCL10) peaking at 6 h, resembling the kinetics observed in mice. All patients developed de novo T-cell responses against the vaccine antigens.
- In patient 1, who had no T cells against NY-ESO-1 at baseline, cell counts of de novo induced NY-ESO-1-specific T cells four weeks after the last immunization reached the same range as those against immune-dominant HLA-class-I-restricted peptides from cytomegalovirus, Epstein–Barr and influenza viruses. HLA-B35 NY-ESO-1 dextramer analysis of blood samples showed a rapid induction of antigen-specific CD8+T cells within two weeks of starting treatment, increasing with subsequent vaccinations. Moreover, the pre-existing T-cell response against tyrosinase in this patient was augmented. Imaging before and after vaccination showed regression of a suspected metastatic thoracic lymph node lesion (data not shown).
- Patient 2, whose metastases were surgically removed before vaccination, experienced induction of CD4+T-cell responses against NY-ESO-1 and MAGE-A3 and was tumor-free at the time of this report (seven months after the start of vaccination).
- In patient 3, a strong NY-ESO-1-specific HLA-Cw03-directed de novoT-cell response and a weaker response against MAGE-A3 were induced. This patient previously received various treatments and had eight lung metastases at recruitment, which remained clinically and radiologically stable.
RNA Vaccine in Prostate Cancer (PCa)
CV9103 is an mRNA vaccine targeting 4 antigens: prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), prostate stem cell antigen (PSCA), and six-transmembrane epithelial antigen of the prostate 1 (STEAP1). In healthy men, these antigens are frequently and almost exclusively expressed in the prostate, and overexpressed in prostate cancer; with the exception of PSMA which is also overexpressed in the tumor-neovasculature of other cancers. (Unlike LXP-RNA, which is comprised of lipid nanoparticles, CV9103 is a mixture of free mRNA and mRNA complexed with protamine.]
CV9103 encodes full-length antigens and thus has the advantage to induce an immune response against all epitopes contained in the target protein without HLA-restrictions. This, and the inclusion of multiple antigens, was developed to reduce the risk of tumor immune escape due to loss of expression of individual antigens [28], to increase the clinical efficacy by inducing a broader immune response and to provide immune responses against antigens present in the individual tumor in a higher number of patients.
In a phase I/II study of intradermally-administered CV9103 to men with CRPC (castration-resistant prostate cancer):
- Safety: Treatment-related AEs (adverse events) were experienced by 39 (89 %) patients, with a total of 282 related AEs reported. The most frequent treatment-related side effects were injection site erythema and injection site reaction in 27 (61 %) and 21 (48 %) patients, respectively. Fatigue (18 %), pyrexia (16 %), chills (11 %), and influenza-like illness (11 %) were also frequently reported (Table 2). A trend for a dose-dependency of these AEs was not observed. In general, AEs were manageable and resolved upon therapy.
- Immunologic Response:
- Quantitative analysis of ELISpot, ICS (intracellular cytokine staining), and tetramer staining assays revealed that CV9103 was able to induce both CD4 and CD8 T cell responses. The percentage of high-dose patients with increased frequencies of T cells releasing cytokines in an antigen-specific manner after vaccination was 27 % for CD8 cells and 42 % for CD4 cells. In these patients, numbers of antigen-specific cells against at least one antigen were increased compared with baseline at least at one time point. Twelve percent of patients showed both CD8 and CD4 responses, while 55 % of patients had either CD4 or CD8 responses as assessed by ELISpot and ICS.
- Forty-two percent of immunologically responding patients responded to only 1 antigen (single responders), whereas 31 % recognized two, 19 % three and 8 % all four antigens, resulting in 58 % multiple responders (patients responding against more than 1 antigen).
- Therapeutic Activity:
- To assess a potential impact of CV9103 on the clinical course of prostate carcinoma patients, PSA progression-free survival (PSA-PFS; interval from the first vaccination to PSA progression according to Prostate Cancer Working Group 2 [PCWG2] criteria) was measured in 38 patients at 1280 μg CV9103. Median PSA PFS was 1.8 month [95 % CI: 1.4; 3.2], the 6 months PSA PFS rate was 15.9 %;
- One patient with lymph node metastases and a Gleason score of 9 at diagnosis had a confirmed PSA response with a maximum decline of PSA >80 % from baseline in week 23 without any supplementary anti-cancer therapy (Additional file 3shows this case in detail). After an initial rise in week 7, a substantial decline of PSA level was observed in this patient, which was confirmed another 4 weeks later (Baseline PSA: 3.96 μG/L; Week 7: 9.75 μG/L, Week 23: 1,36 μG/L, confirmed one month later: 0,96 μG/L). No further PSA follow up values were available. The patient died 30 months after start of CV9103 treatment. Immunologically, this patient had a cellular immune response to PSA with a 9-fold increase of PSA-specific CD4 T cells detected by ICS in week 9;
- Median overall survival (OS) for all 44 patients was 29.3 months [95 % CI: 21.2; n.a.], in the subgroup of patients with metastatic disease (n = 36), median OS was 31.4months
- During the course of vaccination, no change in the frequency of regulatory T cells could be detected in any of the patients.
- Patients exhibiting an immunological response to more than one antigen (multiple responders) had longer survival times than non-responding patients or patients responding to only one antigen (HR = 41, [95 % CI: 0.17; 0.95], p = 0.017). Kaplan-Meier survival estimates were highest in patients responding to 3 or 4 antigens of CV9103.
![Kaplan-Meier plots of overall survival by immunological responses. (a) Kaplan-Meier survival curves of metastatic patients evaluable for immune responses (n = 26), including immunological responders (n = 20) and non-responders (n = 6). The Hazard Ratio of survival for responders versus non-responders was 0.30, [95 % CI: 0.08; 1.15], p = 0.09. (b) Kaplan-Meier survival curves of metastatic patients evaluable for immune responses (n = 26), evaluated according to the number of CV9103 antigens the patients responded to. The Hazard Ratio was 0.41, [95 % CI: 0.17; 0.95], and p = 0.017. http://jitc.biomedcentral.com/articles/10.1186/s40425-015-0068-y](http://blogs.shu.edu/cancer/files/2016/06/Prostate-RNA-Vaccine-Survival.gif)
Kaplan-Meier plots of overall survival by immunological responses. (a) Kaplan-Meier survival curves of metastatic patients evaluable for immune responses (n = 26), including immunological responders (n = 20) and non-responders (n = 6). The Hazard Ratio of survival for responders versus non-responders was 0.30, [95 % CI: 0.08; 1.15], p = 0.09. (b) Kaplan-Meier survival curves of metastatic patients evaluable for immune responses (n = 26), evaluated according to the number of CV9103 antigens the patients responded to. The Hazard Ratio was 0.41, [95 % CI: 0.17; 0.95], and p = 0.017. http://jitc.biomedcentral.com/articles/10.1186/s40425-015-0068-y
It would also be prudent to combine this approach with checkpoint inhibitors (e.g., ipilimumab and nivolumab or pembrolizumab) so that the immune responses induced by the RNA vaccines are not abrogated by the tumor.