Celgene and Lycera entered into a $105MM collaboration on its RORϒ agonists for the treatment of cancer, as well as LYC-30937, an oral gut-directed ATPase modulator now in early-stage clinical studies. LYC-30937 is designed to treat IBD without global immune suppression.
The deal was triggered by data released by Lycera in February regarding the potential of its oral compounds to modulate the activity of T-helper cells and cytotoxic T-cells that express IL-17. The molecules are the only disclosed agonists of RORgammaT. RORgammaT, also known as retinoic acid-related (RAR) orphan receptor C thymus-specific isoform, drives the activation and differentiation of CD4+ and CD8+ cells into IL-17-producing T helper type 17 (Th17) cells and cytotoxic Tc17 cells.
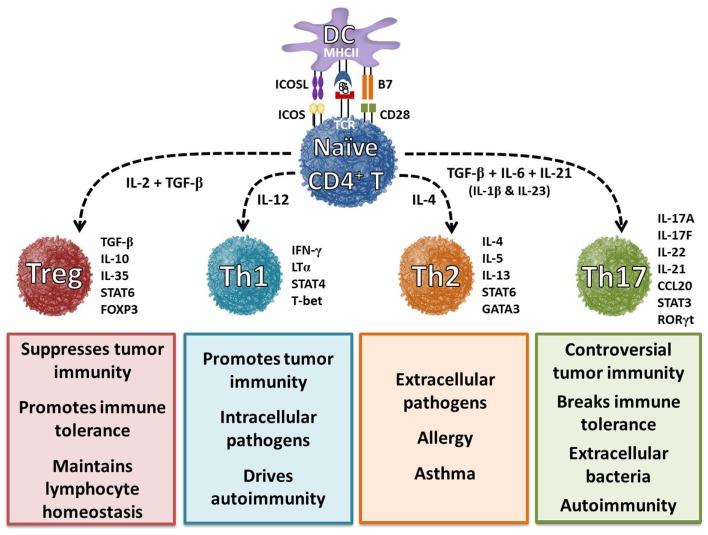
What are Th17 Cells?
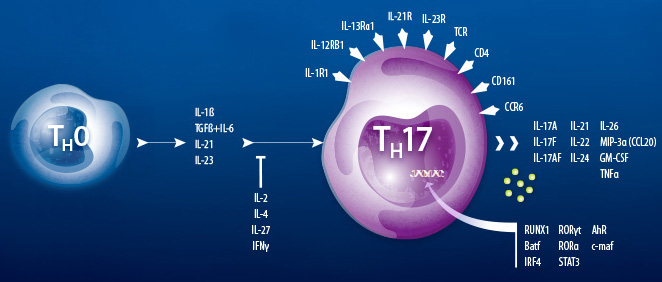
Th17 cells are a new lineage of T-helper cells identified in 2000. Functionally, Th17 cells play a role in host defense against extracellular pathogens by mediating the recruitment of neutrophils and macrophages to infected tissues. Moreover, it has become evident that aberrant regulation of Th17 cells may play a significant role in the pathogenesis of multiple inflammatory and autoimmune disorders.
The Master Regulator of Th17 Differentiation
Differentiation of Th17 cells is controlled by a “master-regulator” transcription factor, ROR gamma t, which directs a specific and heritable gene expression profile. ROR gamma t was initially identified as a thymus-specific isoform of ROR gamma, and later, it was discovered that ROR gamma t is also expressed in Th17 cells. ROR gamma t deficiency results in diminished Th17 activity and severely reduced expression of IL-17.
Although Th17 cells eradicate tumors when transferred into mice, they also function as regulatory cells with the capacity to suppress antitumor immunity. Two distinct mechanisms that sustain their immunosuppressive nature have been identified. One, Th17 cells are capable of converting into Treg cells (i.e., plasticity); two, Th17 cells release immunosuppressive adenosine upon TGF-β-dependent ectonucleotidase expression as illustrated in below.
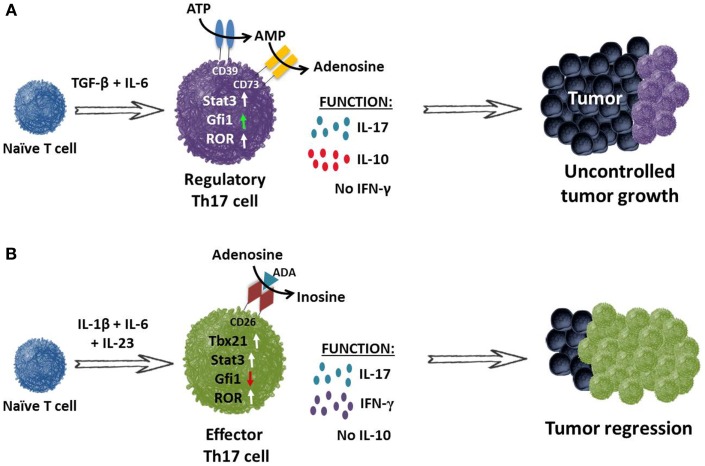
TGF-β induces Th17 cells to express ectonucleotidases and release immunosuppressive adenosine. (A) Th17 cells programed with TGF-β and IL-6 fail to secrete IFN-γ but do secrete IL-17A and IL-10. These cells expressed nominal amounts of Gfi1 (growth factor independent protein 1 – repressor of ectonucleotidase), resulting in CD39 and CD73 ectonucleotidase expression on their cell surface. CD39 and CD73 convert ATP to immunosuppressive adenosine, thereby contributing to the inhibition of antitumor immunity. (B) Conversely, programing CD4+ T cells in the absence of TGF-β but presence of IL-1β, IL-6 and IL-23 supports the generation of Th17 cells that secrete IL-17A and express RORγt and STAT3. Moreover, these cells also express transcription factor Tbx21 and co-secrete IL-17 and IFN-γ but not the immunosuppressive cytokine IL-10. These inflammatory Th17 cells also express increased Gfi1. It is also possible that they express CD26, which facilitates the conversion of adenosine to inosine upon binding of adenosine deaminase (ADA). These infused cells promote the activation of CD8+ effector T cells and cooperate to mediate tumor regression in an IFN-γ and IL-17A-dependent manner.
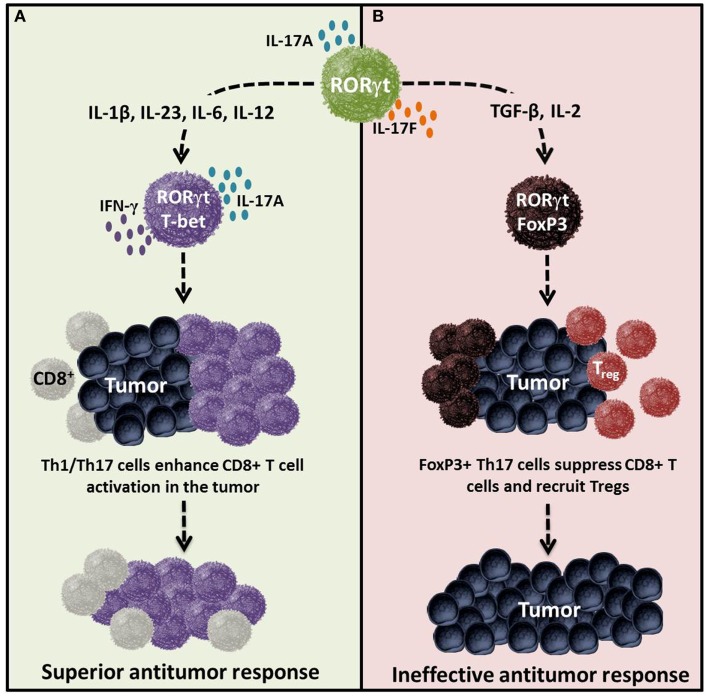
Th17 Plasticity – Cytokines determine the effector versus regulatory nature of Th17 cells in tumor immunity. Cytokines and costimulatory molecules distinctly transform Th17 cells into either an effector or regulatory phenotype, which in turn regulates immunity to self/tumor tissue. (A) Effector Th17 cells activated with IL-1β, IL-23, IL-6, IL-12 and/or ICOS agonist are poly-functional and are capable of mediating potent antitumor immunity. (B) Regulatory Th17 cells programed with cytokines such as TGF-β, IL-2, and/or CTLA4 can dampen their function and persistence, thereby potentially reducing their capacity to kill tumors. Regulatory Th17 cells likely do not foster the induction or cooperation of CTLs to the malignant site. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4060300/figure/F3/
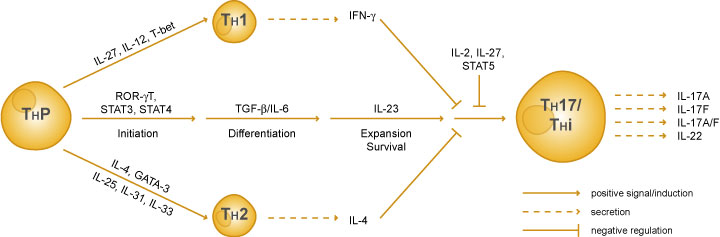
Modulating Th17 and Tc17 Cells
Th17 and Tc17 are effector cells that promote inflammation, adaptive immunity and autoimmunity by producing IL-17 and other inflammatory cytokines such as IL-21.
In both murine and naïve human T cells that were differentiated in vitro into Th17 cells, the addition of an RORgammaT agonist led the cells to produce significantly more GM-CSF, IL-17A and IL-17F than a dimethyl sulfoxide vehicle did. The study also demonstrated target specificity, because the agonist did not significantly affect levels of IFN gamma, an antitumor cytokine that is not controlled by RORgammaT.
In a second in vitro study, Lycera showed the agonist led to an increase in Th17 and Tc17 cells as measured by IL-17 mRNA, and a decrease in Treg cells, which suppress activation of the immune system.
Potential of Increased Levels of Th17 and Tc17 in Cancer Immunotherapy
By increasing the activity of anti-tumor T-helper and cytotoxic T-cells, the RORg agonists can lead to a more robust antitumor response. And, by reducing the expression of Treg cells, they can block one of the mechanisms by which cancer cells escape immune attack. “What you’d love to do is have more effector cells and fewer regulatory cells. That would in theory have more of an antitumor effect and a less immunosuppressive environment,” said founder and CSO Gary Glick.
Interestingly, studies have shown that T-cells induced by the RORg agonists express about half as much PD-1 as those treated with vehicle. Since PD-1 binding by PD-L1 triggers abrogation of the antitumor response, the expression of less PD-1 represents a preferred phenotype of tumor T cells with respect to cancer cell killing.
Cells that were differentiated with the agonist proliferated at the same levels whether or not PD-L1 was added. In contrast, cells differentiated without the agonist proliferated half as much with the ligand as without it. “Cells differentiated in the presence of the agonist have a reduced sensitivity to checkpoint inhibition,” noted Glick.